Abstract
Aims
In the present phase II randomized study, two different schedules of ondansetron were investigated as rescue antiemetic treatment for delayed emesis related to moderately emetogenic chemotherapy (MEC).
Materials and methods
Patients scheduled to receive a first course of MEC were randomized to ondansetron 8 mg intramuscularly (arm A) or ondansetron 16 mg orally (arm B) as rescue antiemetic treatment for delayed emesis. Efficacy and safety evaluation was performed from days 2 to 6 through the administration of a diary plus a questionnaire in which the emetic episodes and the use of the assigned rescue treatment were recorded. All patients received standard prophylaxis for delayed emesis with oral dexamethasone 8 mg daily for 4 days starting on day 2.
Results
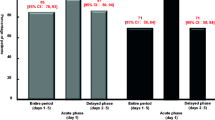
Eighty-nine patients were enrolled into the study, of whom 44 were randomized to arm A and 45 to arm B. Twenty-two patients in each arm developed grade 1–2 delayed nausea/vomiting, all of which recurred to the rescue study treatment. Oral ondansetron resulted superior to intramuscular ondansetron in terms of complete response for nausea (77.3% vs 40.9%, respectively, p = 0.01) and vomiting (81.8% vs 31.8%, respectively, p = 0.001). Both schedules resulted to be very well tolerated, and no differences in toxicity were observed between the two arms of treatment. Furthermore, personal satisfaction about the use of the assigned rescue study medication was significantly higher in arm B.
Conclusions
Due to its high efficacy and excellent tolerability, oral ondansetron is an important option in the management of MEC-related delayed emesis refractory to standard antiemetic prophylaxis.


Similar content being viewed by others
References
Antiemetic Subcommitee of the Multinational Association of Supportive Care in Cancer (1998) Prevention of chemotherapy- and radiotherapy induced emesis: results of the Perugia Consensus Conference. Ann Oncol 9:811–819
Demoor C, Cohen L, Eisenberg PD, Grunberg SM, Kim YJ, Rubenstein EB (2003) Oncologists’ compliance with antiemetic guidelines (AEG) and outcomes of patients (pts) receiving emetogenic chemotherapy (CT). Proc Am Soc Clin Oncol 22: Abstract 2924
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S, 99-04 Palonosetron Study Group (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98:2473–2482
Gandara DR, Roila F, Warr D, Edelman MJ, Perez EA, Gralla RJ (1998) Consensus proposal for 5HT3 antagonists in the prevention of acute emesis related to higly emetogenic chemotherapy. Support Care Cancer 6:237–243
Gralla RJ, de Wit R, Herrstedt J, Carides AD, Ianus J, Guoguang-Ma J, Evans JK, Horgan KJ (2005) Antiemetic efficacy of the neurokinin-1 antagonist aprepitant plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin. Cancer 104:864–868
Gralla RS, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, Clark-Snow R, Gill DP, Groshen S, Grunberg S, Koeller JM, Morrow GR, Perez EA, Sliber JH, Pfister DG (1999) Recommendation for the use of antiemetics. Evidence-based report by the American Society of Clinical Oncology. J Clin Oncol 17:2971–2994
Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14:1570–1577
Grunberg SM, Hesketh PJ (1993) Control of chemotherapy-induced emesis. N Engl J Med 329:1790–1796
Herrstedt J, Sigsgaard T, Boesgaard M, Jensen TP, Dombernowsky P (1993) Ondansetron plus metopimazine compared with ondansetron alone in patients receiving moderately emetogenic chemotherapy. N Engl J Med 328:1076–1080
Kris MG, Gralla RJ, Clark RA, Tyson LB, O’Connel JP, Wertheim MS, Kelsen DP (1985) Incidence, cause, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol 3:1379–1384
Kris MG, Roila F, De Mulder PHM, Marty M (1998) Delayed emesis following anticancer chemotherapy. Support Care Cancer 6:228–232
Kris MG, Hesketh PJ, Somerfiel MR, Feyer P, Clark-Snow R, Koeller JM, Morow CR, Chinnery LM, Chesney MJ, Gralla RJ, Grunberg SM (2006) American Society of Clinical Oncology guideline for antiemetics in oncology. Update 2006. J Clin Oncol 24:2932–2947
Lebeau B, Depierre A, Giovannini M, Rivière A, Kaluzinski L, Votan B, Hédouin M, d’Allens H (1997) The efficacy of combination of ondansetron, mehylprednisolone and metopimazine in patients previously uncontrolled with a dual antiemetic treatment in cisplatin-based chemotherapy. Ann Oncol 8:887–892
National Cancer Institute. Common toxicity criteria version 3.0. Available at: http://ctep.cancer.gov/forms/CTCAEv3.pdf. Accessed August 3, 2007
National Comprehensive Cancer Network. Antiemesis (2004) Clinical practice guidelines in oncology. J Natl Compr Cancer Netw 2:470–490
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Antiemetics, V.1.2007. Available at: http://www.nccn.org/professionals/physician_gls/PDF/antiemetics.pdf. Accessed August 3, 2007
Perez EA, Hesketh PJ, Gandara DR (1991) Serotonin antagonists in the management of cisplatin-induced emesis. Semin Oncol 18(Suppl 3):73–80
Roila F, Hesketh PJ, Herrstedt J, Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (2006) Antiemetic subcommittee of prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 17:20–28
Schwartzberg L (2006) Chemotherapy-induced nausea and vomiting: state of the art in 2006. J Support Oncol 4(Suppl 1):3–8
The Italian Group for Antiemetic Research (2000) Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med 342:1554–1549
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fabi, A., Ciccarese, M., Metro, G. et al. Oral ondansetron is highly active as rescue antiemetic treatment for moderately emetogenic chemotherapy: results of a randomized phase II study. Support Care Cancer 16, 1375–1380 (2008). https://doi.org/10.1007/s00520-008-0438-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-008-0438-9