Abstract
Background
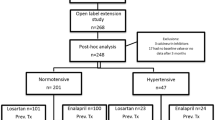
A previous subgroup analysis of a 12-week, double-blind study demonstrated that losartan significantly lowered proteinuria versus placebo and amlodipine and was well tolerated in children (1–17 years old) with proteinuria secondary to Alport syndrome. The present subgroup analysis of the open-label, extension phase of this study assessed the long-term efficacy and tolerability of losartan versus enalapril.
Methods
Patients who had completed the double-blind study were re-randomized to losartan or enalapril and followed for proteinuria and renal function for up to 3 years.
Results
Twenty-seven patients with Alport syndrome were randomized to losartan (0.44-2.23 mg/kg/day; n = 15) or enalapril (0.07-0.72 mg/kg/day; n = 12). The least-squares (LS) mean percent change from week 12 in urinary protein to creatinine ratio (UPr/Cr was +1.1 % in the losartan group versus a further 13.9 % reduction in the enalapril group (GMR [95 % CI] = 1.2 [0.7, 2.0]); the LS mean change from week 12 in estimated glomerular filtration rate (eGFR) was −6.4 ml/min/1.73 m2 in the losartan group versus −9.1 ml/min/1.73 m2 in the enalapril group. The adverse event incidence was low and comparable in both treatment groups.
Conclusions
In children with proteinuria secondary to Alport syndrome, losartan maintained proteinuria reduction, and enalapril produced a further proteinuria reduction over the 3-year study period. Both agents were generally well tolerated.


Similar content being viewed by others
References
Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Kashtan CE, Gubler MC (2009) Inherited glomerular diseases. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N (eds) Pediatric nephrology. Springer, Berlin Heidelberg New York, pp 622–628
Gross O, Kashtan CE (2009) Treatment of Alport syndrome: beyond animal models. Kidney Int 76:599–603
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345:861–869
Fernandez-Andrade C, Russo D, Iversen B, Zucchelli P, Aranda P, Guerra L, Casado S (1998) Comparison of losartan and amlodipine in renally impaired hypertensive patients. Kidney Int Suppl 68:S120–S124
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329:1456–1462
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345:851–860
Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P (1996) Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 334:939–945
GISEN The Group (1997) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 349:1857–1863
Kashtan CE, Ding J, Gregory M, Gross O, Heidet L, Knebelmann B, Rheault M, Licht C (2012) Clinical practice recommendations for the treatment of Alport syndrome: a statement of the Alport Syndrome Research Collaborative. Pediatr Nephrol. doi:10.1007/s00467-012-2138-4
Webb NJ, Lam C, Shahinfar S, Strehlau J, Wells TG, Gleim GW, Le Bailly DT (2011) Efficacy and safety of losartan in children with Alport syndrome–results from a subgroup analysis of a prospective, randomized, placebo- or amlodipine-controlled trial. Nephrol Dial Transplant 26:2521–2526
Webb NJ, Lam C, Loeys T, Shahinfar S, Strehlau J, Wells TG, Santoro E, Manas D, Gleim GW (2010) Randomized, double-blind, controlled study of losartan in children with proteinuria. Clin J Am Soc Nephrol 5:417–424
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Webb NJA, Shahinfar S, Wells TG, Massaad R, Gleim GW, Santoro EP, Sisk CM, Lam C (2012) Losartan and enalapril are comparable in reducing proteinuria in children. Kidney Int. doi:10.1038/ki.2012.210
National High Blood Pressure Education Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Moller K, Wigger M, Peruzzi L, Mehls O, Schaefer F (2009) Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361:1639–1650
Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tonshoff B, Hocker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dotsch J, Muller-Wiefel DE, Hoyer P, Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Muller GA, Weber M (2012) Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81:494–501
Acknowledgments
We are grateful to Kathleen Newcomb (Merck Sharp & Dohme Corp.) for administrative and editorial support of the manuscript.
Disclosures
Dr. Nicholas Webb reports receiving consulting and/or lecture fees and participation on advisory boards alone or in some combination from Merck, Abbott, and Takeda. Dr. Thomas Wells reports receiving consulting fees from Merck and Takeda. Dr. Shahnaz Shahinfar reports receiving consulting fees from Merck. Rachid Massaad, Gilbert W. Gleim, and Christine McCrary Sisk are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, and may hold stock/stock options in the company. Chun Lam is a former employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, and may hold stock/stock options in the company.
Role of the funding source
Merck Sharp & Dohme Corp. a subsidiary of Merck & Co., Inc, Whitehouse Station, NJ, USA, provided all funding and study drugs for this study. The funding source was involved in the design of the study, collection of data, analysis or interpretation of the data, writing or approval of the manuscript, and the decision regarding submission of the manuscript for publication.
Contributions
Nicholas Webb participated in the planning and design of the study, interpretation of results, review and revision of the manuscript, and approved the final version.
Shahnaz Shahinfar participated in the planning and design of the study, interpretation of results, review and revision of the manuscript, and approved the final version.
Thomas Wells participated in the planning and design of the study, interpretation of results, review and revision of the manuscript, and approved the final version.
Rachid Massaad participated in the analyses, provided statistical guidance, reviewed and revised the manuscript, and approved the final version.
Gilbert Gleim participated in the planning and design of the study, review and revision of the manuscript, and approved the final version.
Christine McCrary Sisk participated in interpretation of results, wrote parts of the manuscript, reviewed and revised the manuscript, and approved the final version.
Chun Lam participated in the planning and design of the study, analyses, interpretation of results, wrote parts of the manuscript, reviewed and revised the manuscript, and approved the final version.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is registered on www.clinicaltrials.gov as NCT00568178.
Appendix 1
Appendix 1
Investigators
Chile: Angela Delucchi, Santiago.
Colombia: Pilar M Amado, Bucaramanga; Oscar A. Hernandez, Bogotá; Ricardo Gastelbondo, Bogotá.
Germany: Katalin Dittrich, Erlangen; Juergen Strehlau, Leipzig; Martin Pohl, Freiburg.
Guatemala: Luis F. Arroyo-Garcia, Guatemala City; Randall Lou, Guatemala City.
Hungary: György Reusz, Budapest; László Szabó, Miskolc.
India: Rajiv Aggarwal, Karnataka; Urmila Anandh, Karnataka; Shiela Bhave, Maharashtra; Padmanabha P. Maiya; Karnataka; Kishore Phadke, Karnataka.
Lithuania: Birute Pundziene, Kaunas; Augustina Jankauskiene, Vilnius.
Mexico: Silvestre Garcia, México; Froylán E. Hernández-Lara, Puebla; Ricardo López, Morelos.
Norway: Anna Bjerre, Oslo; Damien Brackman, Bergen.
Panama: Florencio A. McCarthy, Panama City.
Peru: Reyner F. Loza-Munarriz, Lima; Graciela Sakihara-Asato, Lima.
Philippines: Maria-Angeles Marbella, Quezon City; Rosario Cruz, Quezon City.
Puerto Rico: Melvin Bonilla-Felix, San Juan.
Romania: Aurel Bizo, Cluj; Gheorghe Chiriac-Babei, Cluj; Ioan Sabau, Cluj; Victor M. Nanulescu, Cluj.
Russian Federation: Yuri B. Belousov, Moscow; Vladimir V. Dlin, Moscow; Alexey N. Tsygin, Moscow.
Spain: Laura Espinosa-Román, Madrid.
Taiwan: Jeng-Daw Tsai, Taipei; Chi-Hui Cheng, Kweishan Taoyuan.
United Kingdom: William van't Hoff, London; Nicholas Webb, Manchester.
United States: Vimal Chadha, Richmond, Virginia; Vikas R. Dharnidharka, Gainesville, Florida; Robert M. Haws, Marshfield, Wisconsin; Ronald J. Hogg, Temple, Texas; Craig B. Langman, Chicago, Illinois; Kenneth V. Lieberman, Hackensack, New Jersey; Victoria Norwood, Charlottesville, VA; Cynthia G. Pan, Milwaukee, Wisconsin; Howard Trachtman, New Hyde Park, New York; Constancia Uy, Newark, New Jersey
Rights and permissions
About this article
Cite this article
Webb, N.J.A., Shahinfar, S., Wells, T.G. et al. Losartan and enalapril are comparable in reducing proteinuria in children with Alport syndrome. Pediatr Nephrol 28, 737–743 (2013). https://doi.org/10.1007/s00467-012-2372-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-012-2372-9