Abstract
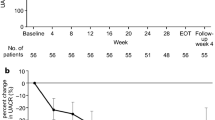
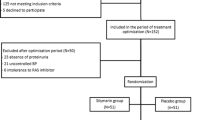
As angiotensin II type 1 receptor blockers (ARBs) may have different antiproteinuric effects in diabetic kidney disease (DKD), we ascertained the albuminuria-reducing effect of fimasartan and losartan in patients with DKD. This was a randomized, multicenter, double-blind, 4-parallel-group, dose-titration, phase III study designed to compare the efficacy of fimasartan and losartan in reducing albuminuria in patients with DKD (NCT02620306). The primary endpoint was the rate of change in albuminuria from baseline to week 24. A total of 341 patients were randomized to different groups. The urinary albumin-to-creatinine ratio (ACR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were not different between the fimasartan and losartan groups at baseline (ACR: 1376.84 vs. 1521.07 mg/gCr, SBP: 154.69 vs. 154.47 mmHg, DBP: 83.96 vs. 83.83 mmHg). However, ACR reduction was significantly larger in the fimasartan group than in the losartan group during the entire study period (% changes in the ACR at 4, 8, 12, and 24 weeks were –23.58, –33.06, –35.00, and –38.13 in the fimasartan group vs. –8.74, –10.17, –14.91, and –19.71 in the losartan group, p < 0.01, respectively). The superior antiproteinuric effect of fimasartan compared to losartan was still significant after adjustment for SBP levels. There were no significant differences in adverse events, including the incidences of estimated glomerular filtration decline and hyperkalemia. This study demonstrates that compared to losartan, fimasartan significantly reduces albuminuria in patients with DKD, even after adjustment for SBP and DBP.



Similar content being viewed by others
References
Hong YA, Ban TH, Kang CY, Hwang SD, Choi SR, Lee H, et al. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean Renal Data System (KORDS). Kidney Res Clin Pract. 2021;40:52–61.
Atkins RC, Briganti EM, Lewis JB, Hunsicker LG, Braden G, Champion de Crespigny PJ, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45:281–7.
Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003;138:542–9.
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76.
de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309–20.
Eijkelkamp WB, Zhang Z, Remuzzi G, Parving HH, Cooper ME, Keane WF, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol. 2007;18:1540–6.
Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115–27.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9.
Parving HH, Hommel E, Smidt UM. Protection of kidney function and decrease in albuminuria by captopril in insulin dependent diabetics with nephropathy. BMJ. 1988;297:1086–91.
Whelton PK, Carey RM, Aronow WS, Casey DE,Jr., Collins KJ, Dennison Himmelfarb C. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104.
Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, et al. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int. 2007;71:1019–27.
Lee SH, Yoo TH, Nam BY, Kim DK, Li JJ, Jung DS, et al. Activation of local aldosterone system within podocytes is involved in apoptosis under diabetic conditions. Am J Physiol Ren Physiol. 2009;297:F1381–1390.
Kim JY, Son JW, Park S, Yoo TH, Kim YJ, Ryu DR, et al. FimAsartaN proTeinuriA SusTaIned reduCtion in comparison with losartan in diabetic chronic kidney disease (FANTASTIC): study protocol for randomized controlled trial. Trials. 2017;18:632.
Lee HY, Oh BH. Fimasartan: a new angiotensin receptor blocker. Drugs. 2016;76:1015–22.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54.
Bakris G, Burgess E, Weir M, Davidai G, Koval S. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy. Kidney Int. 2008;74:364–9.
Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, et al. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther. 2012;34:552–68. 568.e551-559
Kim TW, Yoo BW, Lee JK, Kim JH, Lee KT, Chi YH, et al. Synthesis and antihypertensive activity of pyrimidin-4(3H)-one derivatives as losartan analogue for new angiotensin II receptor type 1 (AT1) antagonists. Bioorg Med Chem Lett. 2012;22:1649–54.
Paik SH, Chi YH, Lee JH, Han HS, Lee KT. Pharmacological profiles of a highly potent and long-acting angiotensin ii receptor antagonist, fimasartan, in rats and dogs after oral administration. Biol Pharm Bull. 2017;40:992–1001.
Kim S, Kim SJ, Yoon HE, Chung S, Choi BS, Park CW, et al. Fimasartan, a novel angiotensin-receptor blocker, protects against renal inflammation and fibrosis in mice with unilateral ureteral obstruction: the possible role of Nrf2. Int J Med Sci. 2015;12:891–904.
Heerspink HJL, Greene T, Tighiouart H, Gansevoort RT, Coresh J, Simon AL, et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: a meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019;7:128–39.
Acknowledgements
This study was supported by Boryung Co., Ltd., Seoul, Korea. The sponsor supported the supply of investigational products, laboratory tests, and expenses incurred for clinical research coordination and data analyses.
We would also like to thank Seung Hwan Han MD, PhD; Jae-Hyun Chang MD, PhD; Bum-Soon Choi MD, PhD; Il-Suk Sohn MD, PhD; Ki Chul Sung MD, PhD; Chan-Duck Kim MD, PhD; Ea Wha Kang MD, PhD; Moo-Yong Rhee MD, PhD; Jae Yoon Park MD, PhD; Cheol Ho Kim MD, PhD; Hu Jun Chin MD, PhD; Seok Yeon Kim MD; Jang-Young Kim MD, PhD; Kihwan Kwon MD, PhD; Dong-Ryeol Ryu MD, PhD; Sung Yun Lee MD, PhD; Eun Hui Bae MD, PhD; Jin-ok Jeong MD, PhD; Jinho Shin MD, PhD; Sang Heon Song MD, PhD; Su-Hyun Kim MD, PhD; Kee Ho Song MD, PhD; Young Min Cho MD, PhD; Dae Jung Kim ME, PhD; Jung Hyun Noh MD, PhD; Sang-Yong Kim MD, PhD; Sung Wan Chun MD, PhD; Hyung-Seop Kim MD, PhD; Ho Cheol Song MD, PhD; and Hyosang Kim MD, PhD for their valuable contributions to this study.
Funding
This study was supported by Boryung Co., Ltd., Seoul, Korea. SP received lecture fees from Pfizer, Boryoung, Hanmi, Daewoong, Donga, Celltrion, Servier, Daiichi Sankyo, and Daewon. SP also received a research grant from Daiichi Sankyo. YJK received consultation fees from Celltrion and lecture fees from Yuhan, Daewoong, Daiichi Sankyo, Bayer, Takeda, and GC Pharma. SJH received lecture fees and research sponsoring from Hanmi, InnoN, Samjin, and Boryung. SGK received lecture fees and research sponsorships from Yuhan, JW Pharma, AstraZeneca, GSK, Fibrogen, Akebia, Omeros, Chinook, MorphoSys, Otsuka and Boryung. JCW received lecture fees and research sponsorships from Chong Kun Dang, Daewoong, Eli Lilly, Yuhan, and Boryung.
Author information
Authors and Affiliations
Contributions
SP made substantial contributions to the study design. THY contributed to manuscript writing and figure creation. All authors contributed equally to the data collection, data interpretation, and literature research and were involved in all stages of manuscript development.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoo, TH., Hong, S.J., Kim, S. et al. The FimAsartaN proTeinuriA SusTaIned reduCtion in comparison with losartan in diabetic chronic kidney disease (FANTASTIC) trial. Hypertens Res 45, 2008–2017 (2022). https://doi.org/10.1038/s41440-022-01028-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01028-6
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
2023 update and perspectives
Hypertension Research (2024)