Abstract
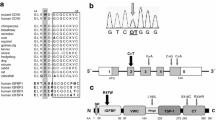
Fgfrl1 (also known as Fgfr5; OMIM 605830) homozygous null mice have thin, amuscular diaphragms and die at birth because of diaphragm hypoplasia. FGFRL1 is located at 4p16.3, and this chromosome region can be deleted in patients with congenital diaphragmatic hernia (CDH). We examined FGFRL1 as a candidate gene for the diaphragmatic defects associated with 4p16.3 deletions and re-sequenced this gene in 54 patients with CDH. We confirmed six known coding single nucleotide polymorphisms (SNPs): c.209G > A (p.Pro20Pro), c.977G > A (p.Pro276Pro), c.1040T > C (p.Asp297Asp), c.1234C > A (p.Pro362Gln), c.1420G > T (p.Arg424Leu), and c.1540C > T (p.Pro464Leu), but we did not identify any gene mutations. We genotyped additional CDH patients for four of these six SNPs, including the three non-synonymous SNPs, to make a total of 200 chromosomes, and found that the allele frequency for the four SNPs, did not differ significantly between patients and normal controls (p ≥ 0.05). We then used Affymetrix Genechip® Mouse Gene 1.0 ST arrays and found eight genes with significantly reduced expression levels in the diaphragms of Fgfrl1 homozygous null mice when compared with wildtype mice—Tpm3, Fgfrl1 (p = 0.004), Myl2, Lrtm1, Myh4, Myl3, Myh7 and Hephl1. Lrtm1 is closely related to Slit3, a protein associated with herniation of the central tendon of the diaphragm in mice. The Slit proteins are known to regulate axon branching and cell migration, and inhibition of Slit3 reduces cell motility and decreases the expression of Rac and Cdc42, two genes that are essential for myoblast fusion. Further studies to determine if Lrtm1 has a similar function to Slit3 and if reduced Fgfrl1 expression can cause diaphragm hypoplasia through a mechanism involving decreased myoblast motility and/or myoblast fusion, seem indicated.

Similar content being viewed by others
References
Ackerman KG, Herron BJ, Vargas SO, Huang H, Tevosian SG, Kochilas L, Rao C, Pober BR, Babiuk RP, Epstein JA, Greer JJ, Beier DR (2005) Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet 1:58–65
Antonius T, van Bon B, Eggink A, van der Burgt I, Noordam K, van Heijst A (2008) Denys-Drash syndrome and congenital diaphragmatic hernia: another case with the 1097G > A(Arg366His) mutation. Am J Med Genet A 146A:496–499
Baertschi S, Zhuang L, Trueb B (2007) Mice with a targeted disruption of the Fgfrl1 gene die at birth due to alterations in the diaphragm. FEBS J 274:6241–6253
Basgul A, Kavak ZN, Akman I, Basgul A, Gokaslan H, Elcioglu N (2006) Prenatal diagnosis of Wolf–Hirschhorn syndrome (4p-) in association with congenital diaphragmatic hernia, cystic hygroma and IUGR. Clin Exp Obstet Gynecol 33:105–106
Begum R, Nur-E-Kamal MS, Zaman MA (2004) The role of Rho GTPases in the regulation of the rearrangement of actin cytoskeleton and cell movement. Exp Mol Med 36:358–366
Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD (2000) Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet 24:438–441
Casaccia G, Mobili L, Braguglia A, Santoro F, Bagolan P (2006) Distal 4p microdeletion in a case of Wolf–Hirschhorn syndrome with congenital diaphragmatic hernia. Birth Defects Res A Clin Mol Teratol 76:210–213
Catela C, Bilbao-Cortes D, Slonimsky E, Kratsios P, Rosenthal N, Te Welscher P (2009) Multiple congenital malformations of Wolf–Hirschhorn syndrome are recapitulated in Fgfrl1 null mice. Dis Model Mech 2:283–294
Clarke NF, Kolski H, Dye DE, Lim E, Smith RL, Patel R, Fahey MC, Bellance R, Romero NB, Johnson ES, Labarre-Vila A, Monnier N, Laing NG, North KN (2008) Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol 63:329–337
Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM (1996) Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet 12:390–397
Dailey L, Ambrosetti D, Mansukhani A, Basilico C (2005) Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 16:233–234
Danhaive O, Lozzi S, D’amico A, Devito R, Boldrini R, Corchia C, Bagolan P, Bertini E (2007) Neonatal-onset nemaline myopathy mimicking congenital diaphragmatic hernia. J Pediatr Surg 42:E19–E22
Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P (1996) Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911–912
Engbers H, van der Smagt JJ, van ‘t Slot R, Vermeesch JR, Hochstenbach R, Poot M (2009) Wolf–Hirschhorn syndrome facial dysmorphic features in a patient with a terminal 4p16.3 deletion telomeric to the WHSCR and WHSCR 2 regions. Eur J Hum Genet 17:129–132
Entezami M, Runkel S, Kunze J, Weitzel HK, Becker R (1998) Prenatal diagnosis of a lethal multiple pterygium syndrome type II. Case report. Fetal Diagn Ther 13:35–38
Flavigny J, Richard P, Isnard R, Carrier L, Charron P, Bonne G, Forissier JF, Desnos M, Dubourg O, Komajda M, Schwartz K (1998) Identification of two novel mutations in the ventricular regulatory myosin light chain gene (MYL2) associated with familial and classical forms of hypertrophic cardiomyopathy. J Mol Med 76:208–214
Fokstuen S, Lyle R, Munoz A, Gehrig C, Lerch R, Perrot A, Osterziel KJ, Geier C, Beghetti M, Mach F, Sztajzel J, Sigwart U, Antonarakis SE, Blouin JL (2008) A DNA resequencing array for pathogenic mutation detection in hypertrophic cardiomyopathy. Hum Mutat 29:879–885
García-Castro M, Reguero JR, Batalla A, Díaz-Molina B, González P, Alvarez V, Cortina A, Cubero GI, Coto E (2003) Hypertrophic cardiomyopathy: low frequency of mutations in the beta-myosin heavy chain (MYH7) and cardiac troponin T (TNNT2) genes among Spanish patients. Clin Chem 49:1279–1285
Griffiths TA, Mauk AG, MacGillivray RT (2005) Recombinant expression and functional characterization of human hephaestin: a multicopper oxidase with ferroxidase activity. Biochemistry 44:14725–14731
Hall C, Flores MV, Murison G, Crosier K, Crosier P (2006) An essential role for zebrafish Fgfrl1 during gill cartilage development. Mech Dev 123:925–940
He YW, Li H, Zhang J, Hsu CL, Lin E, Zhang N, Guo J, Forbush KA, Bevan MJ (2004) The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat Immunol 5:88–97
Hershberger RE, Parks SB, Kushner JD, Li D, Ludwigsen S, Jakobs P, Nauman D, Burgess D, Partain J, Litt M (2008) Coding sequence mutations identified in MYH7, TNNT2, SCN5A, CSRP3, LBD3, and TCAP from 313 patients with familial or idiopathic dilated cardiomyopathy. Clin Transl Sci 1:21–22
Holder AM, Klaassens M, Tibboel D, de Klein A, Lee B, Scott DA (2007) Genetic factors in congenital diaphragmatic hernia. Am J Hum Genet 80:825–845
Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD (2003) Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 78:14387–14393
Howe DT, Kilby MD, Sirry H, Barker GM, Roberts E, Davison EV, Mchugo J, Whittle MJ (1996) Structural chromosome anomalies in congenital diaphragmatic hernia. Prenat Diagn 16:1003–1009
Jandreski MA, Sole MJ, Liew CC (1987) Two different forms of beta myosin heavy chain are expressed in human striated muscle. Hum Genet 77:127–131
Kantarci S, Casavant D, Prada C, Russell M, Byrne J, Haug LW, Jennings R, Manning S, Blaise F, Boyd TK, Fryns JP, Holmes LB, Donahoe PK, Lee C, Kimonis V, Pober BR (2006) Findings from aCGH in patients with congenital diaphragmatic hernia (CDH): a possible locus for Fryns syndrome. Am J Med Genet A 140:17–23
Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, Chassaing N, Lacombe D, Devriendt K, Teebi A, Loscertales M, Robson C, Liu T, MacLaughlin DT, Noonan KM, Russell MK, Walsh CA, Donahoe PK, Pober BR (2007) Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet 39:957–959
Kim I, Moon S, Yu K, Kim U, Koh GY (2001) A novel fibroblast growth factor receptor-5 preferentially expressed in the pancreas(1). Biochim Biophys Acta 1518:152–156
Kim HG, Higgins AW, Herrick SR, Kishikawa S, Nicholson L, Kutsche K, Ligon AH, Harris DJ, Macdonald ME, Bruns GA, Morton CC, Quade BJ, Gusella JF (2007) Candidate loci for Zimmermann-Laband syndrome at 3p14.3. Am J Med Genet A 143:107–111
Laing NG, Wilton SD, Akkari PA, Dorosz S, Boundy K, Kneebone C, Blumbergs P, White S, Watkins H, Love DR et al (1995) A mutation in the alpha tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy NEM1. Nat Genet 10(2):249
Lazjuk GI, Lurie IW, Ostrowskaja TI, Kirillova IA, Nedzved MK, Cherstvoy ED, Silyaeva NF (1980) The Wolf–Hirschhorn syndrome. II. Pathologic anatomy. Clin Genet 18:6–12
Lehtokari VL, Pelin K, Donner K, Voit T, Rudnik-Schöneborn S, Stoetter M, Talim B, Topaloglu H, Laing NG, Wallgren-Pettersson C (2008) Identification of a founder mutation in TPM3 in nemaline myopathy patients of Turkish origin. Eur J Hum Genet 16:1055–1061
Leinwand MJ, Tefft JD, Zhao J, Coleman C, Anderson KD, Warburton D (2002) Nitrofen inhibition of pulmonary growth and development occurs in the early embryonic mouse. J Pediatr Surg 37:1263–1268
Li M, Shuman C, Fei YL, Cutiongco E, Bender HA, Stevens C, Wilkins-Haug L, Day-Salvatore D, Yong SL, Geraghty MT, Squire J, Weksberg R (2001) GPC3 mutation analysis in a spectrum of patients with overgrowth expands the phenotype of Simpson-Golabi-Behmel syndrome. Am J Med Genet 102:161–168
Ma L, Tessier-Lavigne M (2007) Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci 27:6843–6851
Meredith C, Herrmann R, Parry C, Liyanage K, Dye DE, Durling HJ, Duff RM, Beckman K, de Visser M, van der Graaff MM, Hedera P, Fink JK, Petty EM, Lamont P, Fabian V, Bridges L, Voit T, Mastaglia FL, Laing NG (2004) Mutations in the slow skeletal muscle fiber myosin heavy chain gene (MYH7) cause laing early-onset distal myopathy (MPD1). Am J Hum Genet 75:703–708
Mohammadi M, Olsen SK, Goetz R (2005) A protein canyon in the FGF-FGF receptor dimer selects from an à la carte menu of heparan sulfate motifs. Curr Opin Struct Biol 15:506–516
Møller DV, Andersen PS, Hedley P, Ersbøll MK, Bundgaard H, Moolman-Smook J, Christiansen M, Køber L (2009) The role of sarcomere gene mutations in patients with idiopathic dilated cardiomyopathy. Eur J Hum Genet (Epub ahead of print)
Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernández-Martínez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nürnberg P, Reis A, Rauch A (2007) Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 80:550–560
Petrak J, Vyoral D (2005) Hephaestin—a ferroxidase of cellular iron export. Int J Biochem Cell Biol 37:1173–1178
Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, Rayment I, Sellers JR, Fananapazir L, Epstein ND (1996) Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet 13:63–69
Porter JD, Merriam AP, Leahy P, Gong B, Feuerman J, Cheng G, Khanna S (2004) Temporal gene expression profiling of dystrophin-deficient (mdx) mouse diaphragm identifies conserved and muscle group-specific mechanisms in the pathogenesis of muscular dystrophy. Hum Mol Genet 13:257–269
Revencu N, Quenum G, Detaille T, Verellen G, De Paepe A, Verellen-Dumoulin C (2004) Congenital diaphragmatic eventration and bilateral uretero-hydronephrosis in a patient with neonatal Marfan syndrome caused by a mutation in exon 25 of the FBN1 gene and review of the literature. Eur J Pediatr 163:33–37
Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M, EUROGENE Heart Failure Project (2003) Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107:2227–2232
Rieckmann T, Kotevic I, Trueb B (2008) The cell surface receptor FGFRL1 forms constitutive dimers that promote cell adhesion. Exp Cell Res 314:1071–1081
Rieckmann T, Zhuang L, Flück CE, Trueb B (2009) Characterization of the first FGFRL1 mutation identified in a craniosynostosis patient. Biochim Biophys Acta 1792:112–121
Scott DA, Klaassens M, Holder AM, Lally KP, Fernandes CJ (2007) Genome-wide oligonucleotide-based array comparative genome hybridization analysis of non-isolated congenital diaphragmatic hernia. Hum Mol Genet 16:424–430
Sergi C, Schulze BR, Hager HD, Beedgen B, Zilow E, Linderkamp O, Otto HF, Tariverdian G (1998) Wolf–Hirschhorn syndrome: case report and review of the chromosomal aberrations associated with diaphragmatic defects. Pathologica 90:285–293
Slavotinek AM, Moshrefi A, Davis R, Leeth E, Schaeffer GB, Burchard GE, Shaw GM, James B, Ptacek L, Pennacchio LA (2006) Array comparative genomic hybridization in patients with congenital diaphragmatic hernia: mapping of four CDH-critical regions and sequencing of candidate genes at 15q26.1–15q26.2. Eur J Hum Genet 14:999–1008
Sleeman M, Fraser J, McDonald M, Yuan S, White D, Grandison P, Kumble K, Watson JD, Murison JG (2001) Identification of a new fibroblast growth factor receptor, FGFR5. Gene 271:171–182
Soussi-Yanicostas N, Whalen RG, Petit C (1993) Five skeletal myosin heavy chain genes are organized as a multigene complex in the human genome. Hum Mol Genet 2:563–569
Tachdjian G, Fondacci C, Tapia S, Huten Y, Blot P, Nessmann C (1992) The Wolf–Hirschhorn syndrome in fetuses. Clin Genet 42:281–287
Takai Y, Miyoshi J, Ikeda W, Ogita H (2008) Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 9:603–615
Tanno T, Fujiwara A, Sakaguchi K, Tanaka K, Takenaka S, Tsuyama S (2007) Slit3 regulates cell motility through Rac/Cdc42 activation in lipopolysaccharide-stimulated macrophages. FEBS Lett 581:1022–1026
Tapper JK, Zhang S, Harirah HM, Panova NI, Merryman LS, Hawkins JC, Lockhart LH, Gei AB, Velagaleti GV (2002) Prenatal diagnosis of a fetus with unbalanced translocation (4;13)(p16;q32) with overlapping features of Patau and Wolf–Hirschhorn syndromes. Fetal Diagn Ther 17:347–351
Trueb B, Taeschler S (2006) Expression of FGFRL1, a novel fibroblast growth factor receptor, during embryonic development. Int J Mol Med 17:617–620
Trueb B, Zhuang L, Taeschler S, Wiedemann M (2003) Characterization of FGFRL1, a novel fibroblast growth factor (FGF) receptor preferentially expressed in skeletal tissues. J Biol Chem 278:33857–33865
Van Buggenhout G, Melotte C, Dutta B, Froyen G, Van Hummelen P, Marynen P, Matthijs G, de Ravel T, Devriendt K, Fryns JP, Vermeesch JR (2004) Mild Wolf–Hirschhorn syndrome: micro-array CGH analysis of atypical 4p16.3 deletions enables refinement of the genotype-phenotype map. J Med Genet 41:691–698
van Dooren MF, Brooks AS, Hoogeboom AJ, van den Hoonaard TL, de Klein JE, Wouters CH, Tibboel D (2004) Early diagnosis of Wolf–Hirschhorn syndrome triggered by a life-threatening event: congenital diaphragmatic hernia. Am J Med Genet A 127:194–196
Vasudevan PC, Twigg SR, Mulliken JB, Cook JA, Quarrell OW, Wilkie AO (2006) Expanding the phenotype of craniofrontonasal syndrome: two unrelated boys with EFNB1 mutations and congenital diaphragmatic hernia. Eur J Hum Genet 14:884–887
Vasyutina E, Martarelli B, Brakebusch C, Wende H, Birchmeier C (2009) The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc Natl Acad Sci USA 106:8935–8940
Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, Schoenmakers EF, Brunner HG, Veltman JA, van Kessel AG (2004) Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet 36:955–957
Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ (1999) Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21:195–199
Wada H, Nishio H, Kugo M, Waku S, Ikeda K, Takada S, Murakami R, Itoh H, Matsuo M, Nakamura H (1996) Severe neonatal nemaline myopathy with delayed maturation of muscle. Brain Dev 18:135–138
Wiedemann M, Trueb B (2000) Characterization of a novel protein (FGFRL1) from human cartilage related to FGF receptors. Genomics 69:275–279
Wiedemann M, Trueb B (2001) The mouse Fgfrl1 gene coding for a novel FGF receptor-like protein. Biochim Biophys Acta 1520:247–250
Wimplinger I, Morleo M, Rosenberger G, Iaconis D, Orth U, Meinecke P, Lerer I, Ballabio A, Gal A, Franco B, Kutsche K (2006) Mutations of the mitochondrial holocytochrome c-type synthase in X-linked dominant microphthalmia with linear skin defects syndrome. Am J Hum Gene 79:878–889
Yuan W, Rao Y, Babiuk RP, Greer JJ, Wu JY, Ornitz DM (2003) A genetic model for a central (septum transversum) congenital diaphragmatic hernia in mice lacking Slit3. Proc Natl Acad Sci USA 100:5217–5222
Acknowledgments
Anne Slavotinek was generously funded by a K08 grant HD053476-01A1 from the National Institute of Child Health and Development (NICHD) at the National Institutes of Health. Dr Beat Trueb was supported by a Swiss National Science Foundation grant, 3100A0-113806. We are grateful to the Genome Analysis Core Facility and the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco, for their help with the RT-PCR experiments. This publication was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. The findings and conclusions in this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the California Department of Public of Health. We thank the California Department of Public Health Maternal Child and Adolescent Health Division for providing data for these analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
LopezJimenez, N., Gerber, S., Popovici, V. et al. Examination of FGFRL1 as a candidate gene for diaphragmatic defects at chromosome 4p16.3 shows that Fgfrl1 null mice have reduced expression of Tpm3, sarcomere genes and Lrtm1 in the diaphragm. Hum Genet 127, 325–336 (2010). https://doi.org/10.1007/s00439-009-0777-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-009-0777-8