Abstract
The small GTPase Rab5 controls the fusogenic properties of early endosomes through GTP-dependent recruitment and activation of effector proteins. Expression of a GTPase-defective mutant, Rab5(Q79L), is known to cause formation of enlarged early endosomes. The ability of Rab5-GTP to recruit multiple effectors raises the question whether the Rab5(Q79L)-induced giant endosomes simply represent enlarged early endosomes or whether they have a more complex phenotype. In this report, we have addressed this issue by generating a HEp2 cell line with inducible expression of Rab5(Q79L) and performing ultrastructural analysis of Rab5(Q79L)-induced endosomes. We find that Rab5(Q79L) not only induces formation of enlarged early endosomes but also causes enlargement of later endocytic profiles. Most strikingly, Rab5(Q79L) causes formation of enlarged multivesicular endosomes with a large number of intraluminal vesicles, and endosomes that contain both early and late endocytic markers are frequently observed. In addition, we observe defects in the sorting of the EGF receptor and the transferrin receptor through this compartment.










Similar content being viewed by others
References
Bergeland T, Widerberg J, Bakke O, Nordeng TW (2001) Mitotic partitioning of endosomes and lysosomes. Curr Biol 11:644–651
Bergeland T, Haugen L, Landsverk OJ, Stenmark H, Bakke O (2008) Cell-cycle-dependent binding kinetics for the early endosomal tethering factor EEA1. EMBO Rep 9:171–178
Bjerregaard H (2007) Effects of cadmium on differentiation and cell cycle progression in cultured Xenopus kidney distal epithelial (A6) cells. Altern Lab Anim 35:343–348
Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M (1992) The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70:715–728
Ceresa BP, Lotscher M, Schmid SL (2001) Receptor and membrane recycling can occur with unaltered efficiency despite dramatic Rab5(q79l)-induced changes in endosome geometry. J Biol Chem 276:9649–9654
Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62:317–329
Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M (1999) Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol 1:249–252
Dinneen JL, Ceresa BP (2004a) Continual expression of Rab5(Q79L) causes a ligand-independent EGFR internalization and diminishes EGFR activity. Traffic 5:606–615
Dinneen JL, Ceresa BP (2004b) Expression of dominant negative rab5 in HeLa cells regulates endocytic trafficking distal from the plasma membrane. Exp Cell Res 294:509–522
Gillooly DJ, Raiborg C, Stenmark H (2003) Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem Cell Biol 120:445–453
Gorvel JP, Chavrier P, Zerial M, Gruenberg J (1991) rab5 controls early endosome fusion in vitro. Cell 64:915–925
Hirota Y, Kuronita T, Fujita H, Tanaka Y (2007) A role for Rab5 activity in the biogenesis of endosomal and lysosomal compartments. Biochem Biophys Res Commun 364:40–47
McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E (1998) A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol 8:34–45
Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M (1999) Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol 1:376–382
Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M (2000) Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol 151:601–612
Ochi T, Takahashi K, Ohsawa M (1987) Indirect evidence for the induction of a prooxidant state by cadmium chloride in cultured mammalian cells and a possible mechanism for the induction. Mutat Res 180:257–266
Pelkmans L, Burli T, Zerial M, Helenius A (2004) Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118:767–780
Pons V, Luyet PP, Morel E, Abrami L, van der Goot FG, Parton RG, Gruenberg J (2008) Hrs and SNX3 functions in sorting and membrane invagination within multivesicular bodies. PLoS Biol 6:e214
Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H (2002) Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol 4:394–398
Ravikumar B, Imarisio S, Sarkar S, O’Kane CJ, Rubinsztein DC (2008) Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci 121:1649–1660
Rink J, Ghigo E, Kalaidzidis Y, Zerial M (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122:735–749
Roberts RL, Barbieri MA, Pryse KM, Chua M, Morisaki JH, Stahl PD (1999) Endosome fusion in living cells overexpressing GFP-rab5. J Cell Sci 112(Pt 21):3667–3675
Rosenfeld JL, Moore RH, Zimmer KP, Alpizar-Foster E, Dai W, Zarka MN, Knoll BJ (2001) Lysosome proteins are redistributed during expression of a GTP-hydrolysis-defective rab5a. J Cell Sci 114:4499–4508
Rubino M, Miaczynska M, Lippe R, Zerial M (2000) Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J Biol Chem 275:3745–3748
Saksena S, Sun J, Chu T, Emr SD (2007) ESCRTing proteins in the endocytic pathway. Trends Biochem Sci 32:561–573
Semerdjieva S, Shortt B, Maxwell E, Singh S, Fonarev P, Hansen J, Schiavo G, Grant BD, Smythe E (2008) Coordinated regulation of AP2 uncoating from clathrin-coated vesicles by rab5 and hRME-6. J Cell Biol 183:499–511
Simonsen A, Bremnes B, Ronning E, Aasland R, Stenmark H (1998a) Syntaxin-16, a putative Golgi t-SNARE. Eur J Cell Biol 75:223–231
Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H (1998b) EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494–498
Simonsen A, Gaullier JM, D’Arrigo A, Stenmark H (1999) The Rab5 effector EEA1 interacts directly with syntaxin-6. J Biol Chem 274:28857–28860
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol 1985:87–93
Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE (1991) Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol 113:123–135
Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J 13:1287–1296
Stuffers S, Sem Wegner C, Stenmark H, Brech A (2009) Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10:925–937
Tooze SA, Razi M (2009) The essential role of early endosomes in autophagy is revealed by loss of COPI function. Autophagy 5:874–875
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2:107–117
Acknowledgments
We thank Ban-Hock Toh, Jean Gruenberg, Marino Zerial and the Developmental Studies Hybridoma Bank (University of Iowa, USA) for kindly providing antibodies. C.S.W. is a predoctoral fellow of the Norwegian Cancer Society. N.M.P. is a postdoctoral fellow of Regional Health Enterprise South-East. This work was also supported by Functional Genomics (FUGE) grants from the Research Council of Norway to O.B., H.S. and A.B.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00418-009-0660-7
Electronic supplementary material
Below is the link to the electronic supplementary material.
418_2009_643_MOESM1_ESM.pdf
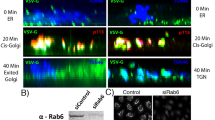
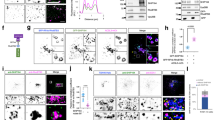
Supplementary Figure 1. HEp2 cells stably expressing the myc-Rab5 QL (called HEp2-Rab5 QL) were grown on coverslips. Rab5 QL expression was induced upon CdCl2-treatment over night. Cells were permeabilized, fixed (3% PFA, 15 min) and stained with anti-myc, anti-EEA1 and anti-CD63 (A), anti-LBPA (B), or anti-Lamp1 (C). Expression of myc-Rab5 QL induced formation of enlarged endosomes recognized by anti-myc. Further, extensive colocalization between myc and EEA1 was observed, implying that both anti-myc as well as anti-EEA1 can be used to visualize the enlarged endosomes. Late endosomal markers such as CD63 (A), LBPA (B) and Lamp1 (C) also associated the enlarged endosomes indicating their heterogenic nature; mixture of early and late endosomes. Scale bar = 5 µm (PDF 1,296 kb)
Rights and permissions
About this article
Cite this article
Wegener, C.S., Malerød, L., Pedersen, N.M. et al. Ultrastructural characterization of giant endosomes induced by GTPase-deficient Rab5. Histochem Cell Biol 133, 41–55 (2010). https://doi.org/10.1007/s00418-009-0643-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-009-0643-8