Abstract
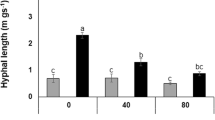
The effects of interactions between Bacillus thuringiensis, a drought-adapted bacterium, and two isolates of Glomus intraradices, an arbuscular mycorrhizal (AM) fungus, on Retama sphaerocarpa, a drought-adapted legume, were investigated. The fungal isolates were an indigenous drought-tolerant and a nonindigenous drought-sensitive isolate. Shoot length and root growth, symbiotic parameters, water transport (in terms of percent relative plant water uptake), and volumetric soil moisture and soil enzymatic activities in response to microbial inoculations were evaluated. Retama plants colonized by G. intraradices plus Bacillus possessed similar shoot length after 30 days from sowing compared with noninoculated Retama plants after 150 days. Inoculation with drought-adapted bacterium increased root growth by 201%, but maximum root development was obtained by co-inoculation of B. thuringiensis and the indigenous G. intraradices. Nodules were formed only in plants colonized by autochthonous AM fungi. Relative water uptake was higher in inoculated than in noninoculated Retama plants, and these inoculants depleted soil water content concomitantly. G. intraradices-colonized Retama reached similar shoot length irrespective of the fungal origin, but there were strong differences in relative water uptake by plants colonized by each one of the fungi. Indigenous G. intraradices-colonized roots (evaluated as functional alkaline phosphatase staining) showed the highest intensity and arbuscule richness when associated with B. thuringiensis. The interactive microbial effects on Retama plants were more relevant when indigenous microorganisms were involved. Co-inoculation of autochthonous microorganisms reduced by 42% the water required to produce 1 mg of shoot biomass. This is the first evidence of the effectiveness of rhizosphere bacterium, singly or associated with AM fungus, in increasing plant water uptake, which represents a positive microbial effect on plants grown under drought environments.




Similar content being viewed by others
References
Allen, EB, Allen, MF (1986) Water relations of xeric grasses in the field: interactions of mycorrhizas and competition. New Phytol 104: 559–571
Augé, RM (2001) Water relations, drought and vesicular–arbuscular mycorrhizal symbiosis. Mycorrhiza 11: 3–42
Azcón, R (1993) Growth and nutrition of nodulated mycorrhizal and non-mycorrhizal Hedysarum coronarium as a result of treatments with fractions from a plant growth-promoting rhizobacteria. Soil Biol Biochem 25: 1037–1042
Barea, JM (1997) Mycorrhiza/bacteria interactions on plant growth promotion. In: Ogoshi, A, Kobayashi, L, Homma, Y, Kodama, F, Kondon, N, Akino, S (Eds.) Plant Growth-Promoting Rhizobacteria, Present Status and Future Prospects. OCDE, Paris, pp 150–158
Barea, JM (2000) Rhizosphere and mycorrhiza of field crops. In: Balázs, E, Galante, E, Lynch, JM, Schepers, JS, Toutant, JP, Werner, D, Werry, PATJ (Eds.) Biological Resource Management: Connecting Science and Policy. Springer-Verlag, Berlin, pp 110–125
Barea, JM, Gryndler, M, Lemanceau, Ph, Schüepp, H, Azcón, R (2002) The rhizosphere of mycorrhizal plants. In: Gianinazzi, S, Schüepp, H, Barea, JM, Haselwandter, K (Eds.) Mycorrhiza Technology in Agriculture: From Genes to Bioproducts. Birkhäuser Verlag, Basel, Switzerland, pp 1–18
Bearden, BN, Petersen, L (2000) Influence of arbuscular mycorrhizal fungi on soil structure and aggregate stability of vertisols. Plant Soil 218: 173–183
Bryla, DR, Duniway, JM (1997) Growth, phosphorus uptake, and water relations of safflower and wheat infected with an arbuscular mycorrhizal fungus. New Phytol 136: 581–590
Caravaca, F, Alguacil, MD, Díaz, G, Roldán, A (2003) Use of nitrate reductase activity for assessing effectiveness of mycorrhizal symbiosis in Dorycnium pentaphyllum under induced water deficit. Commun Soil Sci Plant Anal 34: 2291–2302
Caravaca, F, Figueroa, D, Alguacil, MM, Roldan, A (2003) Application of composted urban residue enhanced the performance of afforested shrub species in a degraded semiarid land. Bioresour Technol 90: 65–70
Galleguillos, C, Aguirre, C, Barea, JM, Azcón, R (2000) Growth promoting effect of two Sinorhizobium meliloti strains (a wild type and its genetically modified derivative) on a non-legume plant species in specific interaction with two arbuscular mycorrhizal fungi. Plant Sci 159: 57–63
García, C, Hernández, MT, Costa, F (1997) Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun Soil Sci Plant Nutr 28: 123–134
Giovannetti, L, Ventura, S, Bazzicalupo, M, Fani, R, Materassi, R (1990) DNA restriction fingerprint analysis of the soil bacterium Azospirillum. J Gen Microbiol 136: 1161–1166
Gordon, SA, Weber, RP (1950) Colorimetric estimation of indole acetic acid. Plant Physiol 26: 192–195
Hall, JA, Pierson, D, Ghosh, S, Glick, BR (1996) Root elongation in various agronomic crops by the plant growth promoting rhizobacteria Pseudomonas putida GR 12-2. Isr J Plant Sci 44: 37–42
Jastrow, JD, Miller, RM (1991) Methods for assessing the effects of biota on soil structure. Agric Ecosyst Environ 34: 279–303
Jeffries, P, Barea, JM (2001) Arbuscular Mycorrhiza—a key component of sustainable plant–soil ecosystems. In: Hock, B (Ed.) The Mycota. Vol. IX, Fungal Associations. Springer-Verlag, Berlin, pp 95–113
Kennedy, AC, Smith, KL (1995) Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 170: 75–86
Koide, RT (1993) Physiology of the mycorrhizal plant. Adv Plant Pathol 9: 33–54
Kramer, PJ, Boyer, JS (1993) Water Relations of Plants and Soils. Academic Press, San Diego, CA
Lachica, M, Aguilar, A, Yañez, J (1973) Análisis foliar, métodos utilizados en la Estación Experimental del Zaidín. An Edafol Agrobiol 32: 1033–1047
Linderman, RG, Davis, EA (2001) Comparative response of selected grapevine rootstocks and cultivars to inoculation with different mycorrhizal fungi. Am J Enol Vitic 52: 8–11
Marulanda, A, Azcón, R, Ruíz-Lozano, JM (2003) Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol Plant 119: 526–533
Masciandaro, G, Ceccanti, B, García, C (1994) Anaerobic digestion of straw and piggery wastewater: II. Optimization of the process. Agrochimica 3: 195–203
Miller, RM, Jastrow, JD (1990) Hierarchy of root and mycorrhizal fungi interactions with soil aggregation. Soil Biol Biochem 22: 579–584
Mitchell, JW, Brunstetter, BC (1939) Colorimetric methods for the quantitative estimation of indole-3-acetic acid. Bot Gaz 100: 802–816
Monzón, A, Azcón, R (1996) Relevance of mycorrhizal fungal origin and host plant genotype to inducing growth and nutrient uptake in Medicago species. Agric Ecosyst Environ 60: 9–15
Monzón, A, Azcón, R (2001) Growth responses and N and P use efficiency of three Alnus species as affected by arbuscular–mycorrhizal colonisation. Plant Growth Regul 35: 97–104
Moreno-Ortego, JL, Falchini, L, Renella, G, Landi, L, Nannipieri, P (1999) Use of the ecological dose (ED50) to assess to toxicity of Cd on soil microbial and biochemical parameters. In: Dick, RP (Ed.) Enzymes in the Environment: Activity Ecology & Applications, Granada, Spain, July 12–15, p 129
Naseby, DC, Lynch, JM (1997) Rhizosphere soil enzymes as indicators of perturbations caused by enzyme substrate addition and inoculation of a genetically modified strain of Pseudomonas fluorescens on wheat seed. Soil Biol Biochem 29: 1353–1362
Phillips, JM, Hayman, DS (1970) Improved procedure of clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55: 159–161
Requena, N, Jeffries, P, Barea, JM (1996) Assessment of natural mycorrhizal potential in a desertified semiarid ecosystem. Appl Environ Microbiol 62: 842–847
Requena, N, Jiménez, I, Barea, JM (1996) Bacteria–mycorrhiza interactions in land restoration. In: Azcón-Aguilar C, Barea JM (Eds.) Mycorrhizas in Integrated Systems. From Genes to Plant Development. COST 821, Bruselas, pp 657–660
Requena, N, Jimenez, I, Toro, M, Barea, JM (1997) Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in Mediterranean semi-arid ecosystems. New Phytol 136: 667–677
Roth, CH, Malicki, MA, Plagge, R (1992) Empirical evaluation of the relationship between soil dielectric constant and volumetric water content as the basis for calibrating soil moisture measurements. J Soil Sci 43: 1–13
Ruíz-Lozano, JM, Azcón, R (1995) Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant 95: 472–478
Ruíz-Lozano, JM, Gómez, M, Azcón, R (1995) Influence of different Glomus species on the time-course of physiological plant-responses of lettuce to progressive drought stress periods. Plant Sci 110: 37–44
Smith, SE, Gianinazzi-Pearson, V (1990) Phosphate uptake and arbuscular activity in mycorrhizal Allium cepa L. Effects of photon irradiance and phosphate nutrition. Aust J Plant Physiol 17: 177–188
Tabatabai, MA (1982) Soil enzymes. In: Page, AL, Miller, EM, Keeney, DR (Eds.) Methods of Soil Analysis. Part 2, 2nd edn, Agronomy Monograph 9. ASA and SSSA, Madison, WI, pp 501–538
Tabatabai, MA, Bremner, JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1: 301–307
Tisserant, B, Gianinazzi-Pearson, V, Gianinazzi, S, Gollotte, A (1993) In planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficient arbuscular mycorrhizal infections. Mycol Res 97: 245–250
Trouvelot, A, Fardeau, JC, Plenchette, C, Gianinazzi, S, Gianinazzi-Pearson, V (1986) Nutritional balance and symbiotic expression in mycorrhizal wheat. Physiol Veg 24: 300
Valdenegro, M, Barea, JM, Azcón, R (2001) Influence of arbuscular–mycorrhizal fungi, Rhizobium meliloti strains and PGPR inoculation on the growth of Medicago arborea used as model legume for re-vegetation and biological reactivation in a semi-arid Mediterranean area. Plant Growth Regul 34: 233–240
Varma, A, Hoock, B (1998) Mycorrhiza: Structure, Function, Molecular Biology and Biotechnology. Springer, Berlin
Vilariño, A, Arines, J (1990) An instrumental modification of Gerdeman and Nicolson's method for extracting VAM fungal spores from soil samples. Plant Soil 121: 211–215
Vivas, A, Biró, B, Ruíz-Lozano, JM, Barea, JM, Azcón, R (2006) Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere 62: 1523–1533
Vivas, A, Marulanda, A, Ruíz-Lozano, JM, Barea, JM, Azcón, R (2003) Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza 13: 249–256
White, I, Knight, JH, Zegelin, SJ, Topp, GC (1994) Comments to ‘considerations on the use of time-domain reflectometry (TDR) for measuring soil water content’ by WR Whalley. J Soil Sci 45: 503–508
Wöhler, I (1997) Auxin-indole derivatives in soils determined by a colorimetric method and by high performance liquid chromatography. Microbiol Res 152: 399–405
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marulanda, A., Barea, J.M. & Azcón, R. An Indigenous Drought-Tolerant Strain of Glomus intraradices Associated with a Native Bacterium Improves Water Transport and Root Development in Retama sphaerocarpa . Microb Ecol 52, 670–678 (2006). https://doi.org/10.1007/s00248-006-9078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9078-0