Abstract
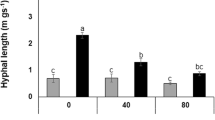
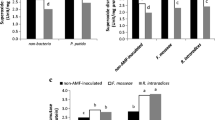
The effects of bacterial inoculation (Bacillus sp.) on the development and physiology of the symbiosis between lettuce and the arbuscular mycorrhizal (AM) fungi Glomus mosseae (Nicol. and Gerd.) Gerd. and Trappe and Glomus intraradices (Schenck and Smith) were investigated. Plant growth, mineral nutrition and gas–exchange values in response to bacterial inoculation after PEG–induced drought stress were also evaluated. In AM plants, inoculation with Bacillus sp. enhanced fungal development and metabolism, measured as succinate dehydrogenase (SDH) and alkaline phosphatase (ALP) activities, more than plant growth. Under non-stressed conditions, G. intraradices colonization increased all plant physiological values to a higher extent when in dual inoculation with the bacterium. Under stress conditions, the bacterium had an important stimulatory effect on G. intraradices development. Under such conditions, the effects of the bacterium on photosynthetic rate, water use efficiency (WUE) and stomatal conductance of lettuce plants differed with the fungus species. Plant-gas exchange was enhanced in G. intraradices- and reduced in G. mosseae-colonized plants when co-inoculated with Bacillus sp. Thus, the effects of each fungus on plant physiology were modulated by the bacterium. Stress was detrimental, particularly in G. intraradices-colonized plants without the bacterium, reducing intra and extraradical mycelium growth and vitality (SDH), as well as plant-gas exchange. Nevertheless, Bacillus sp. inoculation improved all these plant and fungal parameters to the same level as in non-stressed plants. The highest amount of alive and active AM mycelium for both fungi was obtained after co-inoculation with Bacillus sp. These results suggest that selected free-living bacteria and AM fungi should be co-inoculated to optimize the formation and functioning of the AM symbiosis in both normal and adverse environments.




Similar content being viewed by others
References
Amijee F, Tinker PB, Stribley DP (1989) The development of endomycorrhizal root systems.7. A detailed study of the effect of soil phosphorus on colonization. New Phytol 111:435–446
Amora-Lazcano E, Azcón R (1997) Response of sulphur cycling microorganisms to arbuscular mycorrhizal fungi in the rhizosphere of maize. Appl Soil Ecol 6:217–222
Amora-Lazcano E, Vázquez MM, Azcón R (1998) Response of nitrogen transforming microorganisms to arbuscular mycorrhizal fungi. Biol Fertil Soils 27:65–70
Azcón R (1989) Selective interaction between free-living rhizosphere bacteria and vesicular-arbuscular mycorrhizal fungi. Soil Biol Biochem 21:639–644
Azcón R, Azcón-Aguilar C, Barea JM (1978) Effects of plant hormones present in bacterial cultures on the formation and responses to VA mycorrhiza. New Phytol 80:359–369
Azcón-Aguilar C, Barea JM (1985) Effect of soil microorganisms on formation of VA mycorrhizas. Trans Br Mycol Soc 84:536–539
Barea JM (1991) Vesicular-arbuscular mycorrhizae as modifiers of soil fertility, In: Stewart BA (ed) Advances in soil science, vol 15. Springer, New York Berlin Heidelberg, pp 1–39
Barea JM (1997) Mycorrhiza-bacteria interactions on plant growth promotion, In: Ogoshi A, Kobayashi K, Homma Y, Kodama F, Kondo N, Akino S (eds) Plant growth promoting rhizobacteria. Present status and future prospects. OECD, Paris, pp 50–158
Barea JM (2000) Rhizosphere and mycorrhizal of field crops, In: Toutant P, Balazs E, Galante E, Lynch JM, Shepers JS, Werner D, Werry PA (eds) Biological resource managements, connecting science and policy (OECD). INRA and Springer, Berlin Heidelberg New York, pp 110–125
Barea JM, Jeffries P (1995) Arbuscular mycorrhizas in sustainable soil plant systems. In: Varma A, Hock B (eds) Mycorrhiza: structure, function, molecular biology and biotechnology. Springer, Berlin Heidelberg New York, pp 521–559
Barea JM, Gryndler M, Lemanceau P, Schüepp H, Azcón R (2002) The rhizosphere of mycorrhizal plants, In: Gianinazzi S, Schüepp H, Barea JM, Haselwandter K (eds) Mycorrhizal technology in agriculture. From genes to bioproducts. Birkhäuser, Basel, pp 1–18
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Boddington CL, Dodd JC (1998) A comparison of the development and metabolic activity of mycorrhizas formed by arbuscular mycorrhizal fungi from different genera on two tropical forage legumes. Mycorrhiza 8:149–157
Bowen GD, Rovira AD (1999) The rhizosphere and its management to improve plant growth. Adv Agron 66:1–102
Dodd JC, Boddington CL, Rodriguez A, Gonzalez-Chavez C, Mansur I (2000) Mycelium of arbuscular mycorrhizal fungi (AMF) from different genera: form, function and detection. Plant Soil 226:131–151
Duncan DB (1955) Multiple range and multiple F-tests. Biometrics 11:1–42
Gale J, Zeroni M (1985) The cost to plants of different strategies of adaptation to stress and the alleviation of stress by increasing assimilation. Plant Soil 89:57–67
Garbaye J (1994) Helper bacteria: a new dimension to the mycorrhizal symbiosis. New Phytol 128:197–210
Giovannetti L, Ventura S, Bazzicalupo M, Fani R, Materassi R (1990). DNA restriction fingerprint analysis of the soil bacterium Azospirillum. J Gen Microbiol 136:1161–1166
Guillemin JP, Orozco MO, Gianinazzi-Pearson V, Gianinazzi S (1995) Influence of phosphate fertilization on fungal alkaline phosphatase and succinate dehydrogenase activities in arbuscular mycorrhiza of soybean and pineapple. Agric Ecosyst Environ 53:63–69
Hetrick BAD (1989) Acquisition of phosphorus by VA mycorrhizal fungi and the growth responses of their host plants. In: Boddy L, Marchand RM, Read DJ (eds) Nitrogen, phosphorus and sulphur utilization by fungi. Cambridge University Press, Cambridge, pp 205–226
Hewitt EJ (1952) Sand and water culture methods used in the study of plant nutrition. Tech Comm 22. Commonwealth Agricultural Bureaux, Farnham Royal, UK
Jeffries P, Barea JM (1994) Biogeochemical cycling and arbuscular mycorrhizas in the sustainability of plant-soil systems. In: Gianinazzi S, Schüepp H (eds) Impact of arbuscular mycorrhizas on sustainable agriculture and natural ecosystems. Birkhäuser, Basel, pp 101–115
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–586
Jones PCT, Mollinson JE (1948) A technique for the quantitative estimation of soil micro-organisms. J Gen Microbiol 2:54–69
Lachica M, Aguilar A, Yañez J (1973) Análisis foliar, métodos utilizados en la Estación Experimental del Zaidín. Anal Edafol Agrobiol 32:1033–1047
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–147
Long SP, Hallgren JE (1987) Measurements of CO2 assimilation by plants in the field and the laboratory. In: Coombs J, Hall DO, Long SP, Scurlock JMO (eds) Techniques in bioproductivity and photosynthesis, 2nd edn. Pergamon, Oxford, pp 62–94
Newman EI (1966) A method of estimating the total length of root in a sample. J App Ecol 3:139–145
Olsen SR, Dean LA (1965) Phosphorus. In: Black CA, Evans DD, White JL, Ensminger LE, Clark FE, Dinauer RC (eds) Methods of soil chemical analysis, part 2. American Society of Agronomy, Madison, Wis, pp 1035–1049
Olsson PA, Johansen A (2000) Lipid and fatty acid composition of hyphae and spores of arbuscular mycorrhizal fungi at different growth stages. Mycol Res 104:429–434
Pang PC, Paul EA (1980) Effects of vesicular-arbuscular mycorrhiza on C14 and N15 distribution in nodulated fababeans. Can J Soil 60:241–250
Phillips JM, Hayman DS (1970) Improved procedure of clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:159–161
Requena N, Jimenez I, Toro M, Barea JM (1997) Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in Mediterranean semi-arid ecosystems. New Phytol 136:667–677
Ruiz-Lozano JM, Azcón R (1996) Viability and infectivity of mycorrhizal spores after long term storage in soils with different water potentials. Appl Soil Ecol 3:183–186
Ruiz-Lozano JM, Azcón R (1997) Effect of calcium application on the tolerance of mycorrhizal lettuce plants to polyethylene glycol-induced water stress. Symbiosis 23:9–22
Ruiz-Lozano JM, Azcón R, Gómez M (1995a) Effects of arbuscular-mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ Microbiol 61:456–460
Ruiz-Lozano JM, Gómez M, Azcón R (1995b) Influence of different Glomus species on the time-course of physiological plant responses of lettuce to progressive drought stress periods. Plant Sci 110:37–44
Ruiz-Lozano JM, Collados C, Barea JM, Azcón R (2001) Arbuscular mycorrhizal symbiosis can alleviate drought-induced nodule senescence in soybean plants. New Phytol 151:493–502
Smith FA, Smith SE (1996) Mutualism and parasitism: diversity in function and structure in the ''arbuscular'' (VA) mycorrhizal symbiosis. Adv Bot Res 22:1–43
Smith SE, Gianinazzi-Pearson V (1990) Phosphate uptake and arbuscular activity in mycorrhizal Allium cepa L. Effects of photon irradiance and phosphate nutrition. Aust J Plant Physiol 17:177–188
Tinker PB, Durall DM, Jones MD (1994) Carbon use efficiency in mycorrhizas: theory and sample calculations. New Phytol 128:115–122
Tisserant B, Gianinazzi-Pearson V, Gianinazzi S, Gollotte A (1993) In planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficient arbuscular mycorrhizal infections. Mycol Res 97:245–250
Trouvelot A, Fardeau JC, Plenchett C, Gianinazzi S (1986) Nutritional balance and symbiotic expression in mycorrhizal wheat. Physiol Veg 24:300
Varma A, Hoock B (1998) Mycorrhiza: structure, function, molecular biology and biotechnology. Springer, Berlin Heidelberg New York
Vázquez MM, Bejarano C, Azcon R, Barea JM (2000) The effect of a genetically modified Rhizobium meliloti inoculant on fungal alkaline phosphatase and succinate dehydrogenase activities in mycorrhizal alfalfa plants as affected by the water status in soil. Symbiosis 29:49–58
Vierheilig H, Ocampo JA (1989) Relationship between SDH activity and VA mycorrhizal infection. Agric Ecosyst Environ 29:439–442
Wright DP, Scholes JD, Read DJ (1998) Effects of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant Cell Environ 21:209–216
Acknowledgements
This work was financed by UE (Project ICA4-CT-2001-10057). A.V. is grateful to Fundación Gran Mariscal de Ayacucho (Venezuela) and A.M. to ICI (CICYT, Spain) for their respective research grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vivas, A., Marulanda, A., Ruiz-Lozano, J.M. et al. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza 13, 249–256 (2003). https://doi.org/10.1007/s00572-003-0223-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-003-0223-z