Abstract
Purpose
Therapeutic drug monitoring of cyclosporine minimizes the risk of toxicity and acute rejection after transplantation. Areas under the curve (AUCs) rather than trough concentration-based monitoring are recommended. Population pharmacokinetics (PopPK) modeling and Bayesian estimation seem to be the best way to predict cyclosporine disposition and dose requirements to achieve the therapeutic target in an individual patient because of the possibility of predicting cyclosporine AUC using only a few blood samples. Our objectives were to build a PopPk model for cyclosporine in a Tunisian population of HSCT patients and to develop a Bayesian method for the estimation of individual cyclosporine AUC.
Patients and methods
The PopPk of cyclosporine was studied using nonlinear mixed effects modeling (NONMEM) in 30 patients (index group) receiving cyclosporine on a twice-daily basis. Ten blood samples were collected after steady-state morning cyclosporine dose. Bayesian estimation of individual AUC was made on the basis of three blood concentration measurements in an independent group of 30 patients (test group).
Results
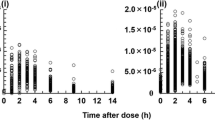
A two-compartment model with first-order absorption and a lag time provided the best fitting. The population mean estimate and interindividual variability from the final model for CL, Ka, Tlag, V1, V2, and Q were 25.4 L/h (CV = 38.72 %), 0.214 h−1(CV = 28.5 %), 0.382 h, 10.9 L (85.73 %), 496 L, and 5 L/h, respectively. Covariates had no discernible effects on cyclosporine pharmacokinetics in our population. Bayesian estimation provided an accurate estimation of AUC, although a bias was observed leading to slight underprediction of AUC (bias −1.03 %). A very satisfactory precision was observed (RMSE 12.07 %).
Conclusion
We report a PopPK model for cyclosporine in Tunisian HSCT patients. Bayesian estimation using only three concentrations provides good prediction of cyclosporine exposure. These tools allow us to routinely estimate cyclosporine AUC in a clinical setting.






Similar content being viewed by others
References
Miller KB, Schenkein DP, Comenzo R, Erban JK, Fogaren T, Hirsch CA, Berkman E, Rabson A (1994) Adjusted-dose continuous-infusion cyclosporin A to prevent graft-versus-host disease following allogeneic bone marrow transplantation. Ann Hematol 68:15–20
Gondo H, Harada M, Taniguchi S, Akashi K, Hayashi S, Teshima T, Takamatsu Y, Eto T, Nagafuji K, Yamasaki K (1993) Cyclosporine combined with methylprednisolone or methotrexate in prophylaxis of moderate to severe acute graft-versus-host disease. Bone Marrow Transplant 2:437–441
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakaka TR (1985) The effect of food on cyclosporine absorption. Transplantation 40:174–176
Dunn CJ, Wagstaff AJ, Perry CM, Plosker GL, Goa KL (2001) Cyclosporin: an updated review of the pharmacokinetic properties, clinical efficacy and tolerability of a microemulsion-based formulation (neoral) 1 in organ transplantation. Drugs 61:1957–2016
Fahr A (1993) Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet 24:472–495
Legg B, Rowland M (1987) Cyclosporin: measurement of fraction unbound in plasma. J Pharm Pharmacol 39:599–603
Trotter JF (1998) Drugs that interact with immunosuppressive agents. Semin Gastrointest Dis 9:147–153
Zaghloul I, Ptachcinski RJ, Burckart GJ, Van Thiel D, Starzel TE, Venkataramanan R (1987) Blood protein binding of cyclosporine in transplant patients. J Clin Pharmacol 27:240–242
Wang CP, Hartman NR, Venkataramanan R, Jardine I, Lin FT, Knapp JE, Starzl TE, Burckart GJ (1989) Isolation of 10 cyclosporine metabolites from human bile. Drug Metab Dispos 17:992–996
Venkataramanan R, Habucky K, Burckart GJ, Ptachcinski RJ (1989) Clinical pharmacokinetics in organ transplant patients. Clin Pharmacokinet 16:134–161
Pamala J, Ng J, Green KG, Rogosheske J, Brundage R (2003) Posttransplant day significantly influences pharmacokinetics of cyclosporine after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 9:304–11
Jebabli N, Klouz A, Bahlous A, Gaies E, Trabelsi S, Lakhal M, Belkehia C (2007) Comparison of three methods for cyclosporine therapeutic monitoring. Transplant Proc 39:2557–2559
Beal SL, Sheiner LB, NONMEM Project Group C255 (1989) Nonlinear mixed effects modelling tool. University of California, San Francisco
Yamaoka K, Nakagawa T, Uno T (1978) Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 6:165–75
Eljebari H, Ben Fradj N, Salouage I, Gaies E, Trabelsi S, Jebabli N, Lakhal M, Ben Othman T, Kouz A (2012) Estimation of abbreviated cyclosporine A area under the concentration-time curve in allogenic stem cell transplantation after oral administration. J Transplant. doi:10.1155/2012/342701
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–12
Sibbald C, Seto W, Taylor T, Saunders EF, Doyle J, Dupuis LL (2008) Determination of area under the whole blood concentration versus time curve after first intravenous cyclosporine dose in children undergoing hematopoietic stem cell transplant: limited sampling strategies. Ther Drug Monit 30:434–8
Hadjibabaie M, Vazirian I, Iravani M, Moosavi SA, Alimoghaddam K, Ghavamzadeh A, Rezaee S (2011) Development and validation of limited sampling strategies for estimation of cyclosporine area under the concentration-time curve in hematopoietic stem cell transplant patients. Ther Drug Monit 33:673–80
McDonald GB, Shulman HM, Sullivan KM, Spencer GD (1986) Intestinal and hepatic complications of human bone marrow transplantation. Gastroenterology 90:770–784
Schultz KR, Nevill TJ, Toze CL, Corrt T, Currie C, Strong DK, Keown PA (1998) The pharmacokinetics of oral cyclosporin A (Neoral) during the first month after bone marrow transplantation. Transplant Proc 30:1668–1670
Willemze AJ, Cremers SC, Schoemaker RC, Lankester AC, den Hartigh J, Burggraaf J, Vossen JM (2008) Ciclosporin kinetics in children after stem cell transplantation. Br J Clin Pharmacol 66:539–545
Wilhelm AJ, de Graaf P, Veldkamp AI, Janssen JJWM, Huijgens PC, Swart EL (2011) Population pharmacokinetics of ciclosporin in hematopoietic allogenic stem cell transplantation with emphasis on limited sampling strategy. Br J Clin Pharmacol 73(4):553–563. doi:10.1111/j.1365-2125.2011.04116.x
Kim SH, Kim Kyung Im, Yun Hwi-yeol, Kang W, Yoon S-S, Shin WG, Lee Hye-suk, Jung Mi Oh (2010) Population pharmacokinetics of cyclosporine after hematopoietic stem cell transplantation in leukemic patients. Kor J Clin Pharm 20:9–16
Jacobson P, Ng J, Green KG, Rogosheske J, Brundage R (2003) Posttransplant day significantly influences pharmacokinetics of cyclosporine after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 9(5):304–311
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eljebari, H., Gaies, E., Fradj, N.B. et al. Population pharmacokinetics and Bayesian estimation of cyclosporine in a Tunisian population of hematopoietic stem cell transplant recipient. Eur J Clin Pharmacol 68, 1517–1524 (2012). https://doi.org/10.1007/s00228-012-1275-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1275-9