Abstract
Aims/hypothesis
Nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme for NAD+ biosynthesis, exists as intracellular NAMPT (iNAMPT) and extracellular NAMPT (eNAMPT). eNAMPT, secreted from adipose tissue, promotes insulin secretion. Administration of nicotinamide mononucleotide (NMN), a product of the eNAMPT reaction, corrects impaired islet function in Nampt +/− mice. One of its potential targets is the NAD+-dependent deacetylase sirtuin 1. We hypothesised that altered NAMPT activity might contribute to the suppression of islet function associated with inflammation, and aimed to determine whether NMN could improve cytokine-mediated islet dysfunction.
Methods
Acute effects of NMN on cytokine-mediated islet dysfunction were examined in islets incubated with TNFα and IL1β, and in mice fed a fructose-rich diet (FRD) for 16 weeks. Changes in iNAMPT, eNAMPT and inflammation levels were determined in FRD-fed mice.
Results
FRD-fed mice displayed markedly lower levels of circulating eNAMPT, with impaired insulin secretion and raised islet expression of Il1b. NMN administration lowered Il1b expression and restored suppressed insulin secretion in FRD-fed mice. NMN also restored insulin secretion in islets cultured with pro-inflammatory cytokines. The changes in islet function corresponded with changes in key markers of islet function and differentiation. The anti-inflammatory effects of NMN were partially blocked by inhibition of sirtuin 1.
Conclusions/interpretation
Chronic fructose feeding causes severe islet dysfunction in mice. Onset of beta cell failure in FRD-fed mice may occur via lowered secretion of eNAMPT, leading to increased islet inflammation and impaired beta cell function. Administration of exogenous NMN to FRD-fed mice corrects inflammation-induced islet dysfunction. Modulation of this pathway may be an attractive target for amelioration of islet dysfunction associated with inflammation.
Similar content being viewed by others
Introduction
The prevalence of insulin resistance and type 2 diabetes has increased dramatically over the past four decades [1, 2]. However, as a consequence of pancreatic islet beta cell compensation, insulin resistance does not immediately lead to onset of type 2 diabetes [3]. Only when pancreatic islet function becomes impaired, resulting in markedly decreased beta cell mass and suppressed glucose-stimulated insulin secretion (GSIS), does type 2 diabetes fully develop [4]. The precise mechanisms responsible for pancreatic beta cell failure in type 2 diabetes are unclear; however, chronic inflammation plays a crucial role in the process [5, 6]. Type 2 diabetes and insulin resistance are progressive chronic inflammatory states and exposure to pro-inflammatory cytokines, such as IL1β and TNFα, leads to pancreatic beta cell death and suppressed insulin secretion [5–7]. Exposure of pancreatic beta cells to pro-inflammatory cytokines can occur via numerous sources, including secretion by resident or invading immune cells within the islet or as a result of exposure to circulating cytokines [8]. In addition, exposure of islets to pro-inflammatory cytokines or high levels of NEFA and glucose (termed glucolipotoxicity) can induce IL1β and TNFα production within the pancreatic beta cells themselves [5].
Recent studies have demonstrated the importance of nicotinamide phosphoribosyltransferase (NAMPT) for pancreatic beta cell function [9]. NAMPT catalyses the synthesis of nicotinamide mononucleotide (NMN) from nicotinamide and 5-phosphoribosyl-pyrophosphate. NMN in turn is converted to NAD+ by NMN adenylyltransferase [10]. NAMPT exists in two forms, intracellular NAMPT (iNAMPT) and extracellular NAMPT (eNAMPT) [10]. iNAMPT levels are high in brown adipose tissue (BAT), liver and kidney, intermediate in white adipose tissue (WAT), lung, spleen, testes and skeletal muscle, and undetectable in brain and pancreas [9]. eNAMPT, thought to be produced through post-translational modification of iNAMPT, is released into plasma predominantly from adipose tissue, where it catalyses the synthesis of NMN [9]. Due to the absence of iNAMPT in the pancreas, islets rely on circulating eNAMPT as a source of NAD+. Recent studies have highlighted the essential role of eNAMPT for GSIS. Nampt +/− mice display decreased circulating levels of eNAMPT and NMN, are glucose-intolerant and show impaired GSIS. Administration of NMN restores GSIS and improves glucose tolerance in these mice [9]. The mechanism by which NMN corrects impaired GSIS has yet to be fully elucidated, but may involve sirtuin 1, a NAD+-dependent protein deacetylase that promotes GSIS [11–13]. Consistent with this, the phenotype of the beta-cell-specific Sirt1-overexpressing (BESTO) mouse, which shows enhanced GSIS, was found to be lost with increased age. This loss of phenotype with age correlated with reduced plasma NMN levels and was restored by NMN administration [14]. Despite evidence suggesting that eNAMPT/NMN functions positively regulate beta cell function, the role of this pathway in insulin resistance and progression to impaired beta cell function has yet to be described.
Dietary sugar consumption has risen over recent decades, in parallel with increased precedence of the metabolic syndrome and type 2 diabetes, with the widespread use of high-fructose corn syrup contributing significantly to this increase [1, 2, 15]. Correspondingly, in rodent models, consumption of a fructose-rich diet (FRD) can lead to onset of aspects of the metabolic syndrome, including hyperglycaemia, dyslipidaemia and inflammation [16–20]. In humans, elevated intake of sugar-sweetened beverages is associated with an increased risk of the metabolic syndrome and type 2 diabetes [21, 22], whilst fructose consumption has been reported to worsen metabolic abnormalities in obese humans to a greater extent than glucose consumption [23, 24]. Fructose consumption has been linked to development of a pro-inflammatory phenotype in rodent models [18, 19, 25]. To explore the links between eNAMPT/NMN, inflammation and islet function, we investigated whether mice fed an FRD displayed an inflammatory phenotype and impaired islet function, and whether these could be rectified by NMN administration, thus implicating impaired NAMPT function as an underlying mechanism. In addition, we investigated whether NMN was protective against the negative effects of pro-inflammatory cytokines in isolated islets.
Methods
Experimental animals
Male C57BL/6 mice aged 7 weeks (Charles River, Margate, UK) were fed an FRD (60% fructose) (TD.89247; Harlan Laboratories, Wyton, UK) or standard rodent diet (control) for 16 weeks. Subsets of FRD-fed mice were administered NMN (500 mg/kg body weight; i.p.) [9] or an equal volume of vehicle (saline) 16 h prior to tissue sampling to create four groups: (1) control + vehicle (CON+V); (2) control + NMN (CON+NMN); (3) FRD + vehicle (FRD+V); and (4) FRD+NMN. A separate group of 16 week FRD-fed mice were fasted for 24 h prior to tissue sampling. Islets were isolated as described below. Blood was collected in heparin-coated tubes and centrifuged to obtain plasma. BAT and WAT were snap-frozen in liquid nitrogen for analysis of protein levels and gene expression. Animal experiments were conducted in accordance with the Home Office regulations on the Operation of Animals (Scientific Procedures) Act 1986, published by HMSO, London. Animals were maintained on a 12 h light–dark cycle.
Blood chemistry analysis
Blood was collected between 09:00 and 11:00 hours. Plasma glucose (Thermo Electron, Melbourne, VIC, Australia) was analysed via colorimetric assay. Plasma insulin (Mercodia, Uppsala, Sweden) and eNAMPT (Phoenix Pharmaceuticals, Burlingame, CA, USA) concentrations were determined via ELISA.
Islet isolation
Mouse pancreases were digested in 2 ml Hanks’ buffered salt solution (HBSS) containing collagenase P (1 mg/ml) and DNase I (0.15 mg/ml) (both from Roche Diagnostics, Burgess Hill, UK). Islets were hand-picked into RPMI 1640 medium containing 11 mmol/l glucose, supplemented with 10% (vol./vol.) heat-inactivated FBS, 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma Aldrich, Poole, UK). Islets isolated from control mice were cultured for 24 h (37°C; 5% CO2) to allow recovery from isolation. After 24 h, islets were transferred to fresh RPMI medium and incubated with the following treatments for 48 h, before RNA and protein extraction or ex vivo islet function assays. Treatments for 48 h were as follows: IL1β (5 ng/ml) and TNFα (10 ng/ml); palmitate (100 μmol/l); NMN (100 μmol/l); co-incubation with IL1β (5 ng/ml), TNFα (10 ng/ml) and NMN (100 μmol/l); co-incubation with IL1β (5 ng/ml), TNFα (10 ng/ml), NMN (100 μmol/l) and the specific sirtuin 1 inhibitor EX-527 (10 μmol/l); or co-incubation with palmitate (100 μmol/l) and NMN (100 μmol/l). Palmitate was initially dissolved in ethanol before being complexed with BSA (10% wt/vol.). Final concentrations of ethanol (0.5%, vol./vol.) and BSA (1%, wt/vol.) were not toxic to islets. Islets isolated from FRD-fed mice were either picked into RPMI and immediately lysed for RNA extraction, or transferred to RPMI and allowed to recover for 2 h, prior to ex vivo insulin secretion assay.
Insulin secretion ex vivo
For islet insulin secretion assays, batches of eight size-matched islets were pre-incubated for 1 h at 37°C in HBSS containing 3 mmol/l glucose, 10 mmol/l HEPES (pH 7.4) and 0.2% BSA (wt/vol.). For GSIS, islets were incubated for 1 h at 37°C in HBSS (10 mmol/l HEPES [pH 7.4], 0.2% BSA) supplemented with 3 or 17 mmol/l glucose. For leucine-stimulated insulin secretion (LSIS), islets were incubated for 1 h at 37°C in HBSS (3 mmol/l glucose, 10 mmol/l HEPES [pH 7.4], 0.2% BSA) supplemented with 2 or 20 mmol/l leucine. After 1 h, media were collected for determination of insulin levels.
Quantitative RT-PCR
Gene expression was measured by quantitative RT-PCR, using Sybr Green methodology (Invitrogen, Paisley, UK). Gene expression was determined by standard curve methodology, normalised against TaqMan 18S ribosomal RNA (Applied Biosystems, Warrington, UK). Changes in gene expression are represented as fold change relative to 1, where control equals 1. For primer and probe details (Eurogentec, Southampton, UK for all), see electronic supplementary material (ESM) Table 1.
Immunoblotting
Solubilised protein samples (2–10 μg) that had been measured and equalised in each fraction (RC-DC System; Bio-Rad, Hemel Hempstead, UK) were separated by SDS-PAGE and transferred on to polyvinylidene difluoride membrane (GE Healthcare, Amersham, UK). Blots were blocked for 1 h in 5% (wt/vol.) milk protein in Tris-buffered saline/0.1% Tween-20 (vol./vol.) (TBS/T) solution and then incubated overnight in anti-NAMPT (Sigma) or anti-IL1β (Abcam, Cambridge, UK) primary antibody. Detection of bands was achieved by chemiluminescence substrate (SuperSignal West Pico; Pierce, Rockford, IL, USA). Reference protein measurements were made with mouse monoclonal anti-β-actin (clone AC-15) primary antibody in a 3% (wt/vol.) milk/TBS-T solution, at 4°C.
Statistical analysis
Results are expressed as mean±SEM. Statistical comparisons were obtained using GraphPad (GraphPad Software, La Jolla, CA, USA). Statistical differences were calculated using a paired t test or one-way ANOVA followed by Bonferroni’s post test where appropriate.
Results
FRD-fed mice display a pro-inflammatory phenotype with elevated blood glucose
FRD-fed mice developed a pro-inflammatory phenotype, with raised Il1b mRNA in WAT, BAT and islets (Fig. 1a–c), and increased Tnfa (also known as Tnfa) expression in WAT and BAT, but not in islets (Fig. 1d–f). Development of a pro-inflammatory phenotype was associated with raised fasting levels of plasma glucose and insulin in FRD-fed mice compared with control (Fig. 1g, h) and raised fed plasma glucose levels (Fig. 1i). Taken together, mice on an FRD for 16 weeks displayed characteristics of type 2 diabetes, including fasting hyperglycaemia and chronic inflammation, and thus are an attractive model for study of impaired islet function.
FRD-fed mice develop a pro-inflammatory phenotype and hyperglycaemia. a Il1b mRNA levels in WAT, (b) BAT and (c) islets of mice with free access to an FRD. d Tnfa mRNA levels in WAT, (e) BAT and (f) islets of mice with free access to an FRD. g Fasting levels of plasma glucose and (h) insulin. i Fed plasma glucose levels. Data are expressed as mean±SEM; *p < 0.05, **p < 0.01 and ***p < 0.001 for difference between control (CON) and FRD mice
FRD increases iNAMPT abundance in adipose tissue while suppressing circulating eNAMPT concentrations
We next assessed whether increased inflammation and raised plasma glucose were associated with altered iNAMPT abundance in BAT and WAT (predominant sites of synthesis of iNAMPT and release of eNAMPT), or with changes in plasma eNAMPT in FRD-fed mice. Abundance of iNAMPT and expression of Nampt were increased in BAT (Fig. 2a, b) and WAT (Fig. 2c, d) of FRD mice, but despite raised iNAMPT levels, plasma levels of eNAMPT (Fig. 2e) were markedly decreased in FRD-fed mice compared with control.
Altered NAMPT abundance in FRD mice. a Nampt expression and (b) abundance of iNAMPT in BAT of mice with free access to an FRD. c Nampt expression and (d) iNAMPT levels in WAT of mice as above (a, b). e Plasma levels of eNAMPT in FRD-fed mice. Western blots (b, d) are representative (n = 5). *p < 0.05 and **p < 0.01 for difference between control (CON) and FRD mice
Insulin secretion is suppressed in FRD-fed mice
We next assessed whether decreased eNAMPT was associated with impaired islet function. To assess the direct effects of FRD on islet function, insulin secretion in response to glucose or leucine was measured ex vivo in isolated islets. GSIS was markedly reduced by 75 ± 3% (mean±SEM; p < 0.05) (Fig. 3a) in islets isolated from FRD-fed mice compared with control mice. Similarly, LSIS was suppressed (91 ± 7%; p < 0.01) in FRD-fed mice relative to control mice (Fig. 3b). Basal insulin secretion (at 3 mmol/l glucose and 2 mmol/l leucine) was unchanged in FRD-fed mice (Fig. 3a, b). Taken together, decreased eNAMPT in FRD-fed mice was associated with suppressed islet function and increased inflammation.
NMN protects against islet dysfunction in FRD mice. Control (CON) or FRD-fed mice with free access to diet were administered NMN (500 mg/kg body weight) (CON+NMN and FRD+NMN groups) or an equal volume of vehicle (saline) (CON+V and FRD+V groups) 16 h prior to islet isolation. a Comparison of GSIS and (b) LSIS in islets isolated from CON+V (white bars) and FRD+V (black bars) mice. c Comparison of GSIS in islets isolated from CON+V (white bars) and CON+NMN (grey bars) mice, and (d) of LSIS in untreated (white bars) and NMN-treated (grey bars) mice. e Comparison of GSIS and (f) LSIS in islets isolated from CON+V (white bars), FRD+V (black bars) and FRD+NMN (grey bars) mice. *p < 0.05 and **p < 0.01 for differences between CON+V and CON+NMN, and CON+V and FRD+V; † p < 0.05 and ††† p < 0.001 for difference between FRD+V and FRD+NMN
NMN administration protects against islet dysfunction in FRD-fed mice
Since Nampt +/− mice show impaired islet function [9], we reasoned that the suppressed eNAMPT levels observed in FRD-fed mice might play a role in the onset of islet dysfunction in these mice. To further examine this, we investigated whether the reaction product of eNAMPT, NMN, provided protection in vivo against beta cell dysfunction in FRD-fed mice. NMN (500 mg/kg body weight; i.p.) [9, 14] was administered to FRD-fed mice 16 h prior to islet isolation. The effects of NMN administration in vivo on insulin secretion ex vivo were first examined with islets isolated from control mice maintained on a standard diet (CON+NMN group). Islets isolated from CON+NMN mice displayed elevated GSIS (twofold; p < 0.01) (Fig. 3c) compared with CON+V mice (which had been injected with saline). Similarly, LSIS was also modestly but significantly increased in CON+NMN mice (37 ± 8%) compared with CON+V mice (Fig. 3d). NMN had no effect on basal rates of insulin secretion measured at 3 mmol/l glucose or 2 mmol/l leucine (Fig. 3c, d). Significantly, in vivo administration of NMN abolished the suppressive effects of FRD on GSIS and LSIS (Fig. 3e, f). Whereas NMN administration to FRD-fed mice completely restored LSIS ex vivo (Fig. 3f), GSIS was significantly elevated above rates seen in CON+V mice (Fig. 3e) and also greatly exceeded those of CON+NMN mice.
NMN protects against pro-inflammatory cytokine-mediated islet dysfunction
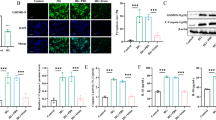
FRD-fed mice developed a pro-inflammatory phenotype. Chronic inflammation through exposure to pro-inflammatory cytokines impairs beta cell function [5, 6]. We hypothesised that the beneficial effects of NMN on FRD-mediated islet dysfunction may occur in part through protection from the effects of pro-inflammatory cytokines. Therefore we investigated whether NMN protected against cytokine-mediated islet dysfunction in islets isolated from mice on a standard diet (control). We first assessed whether NMN affected GSIS and LSIS ex vivo. Culture of islets with NMN (100 μmol/l; 48 h) increased GSIS by 24% (p < 0.05) (Fig. 4a) and greatly enhanced LSIS by 2.7-fold (p < 0.001) (Fig. 4a). To investigate the effects of NMN on pro-inflammatory cytokine-mediated beta cell dysfunction, islets isolated from control mice were incubated with IL1β (5 ng/ml) and TNFα (10 ng/ml), or cultured with IL1β/TNFα plus NMN (100 μmol/l) for 48 h. In islets exposed to IL1β/TNFα, insulin secretion was significantly impaired in response to incubation with 17 mmol/l glucose (39%; p < 0.001) (Fig. 4c) and 20 mmol/l leucine (34%; p < 0.05) (Fig. 4d). However, co-incubation of control islets with NMN completely blocked the effects of IL1β/TNFα, restoring GSIS and LSIS (Fig. 4c, d). IL1β reportedly exerts auto-stimulatory effects, whereby it is able to induce its own production in beta cells, as well as that of TNFα [9]. In agreement with this, Il1b mRNA and the corresponding protein levels, as well as Tnfa mRNA, were increased in islets exposed to IL1β/TNFα (Fig. 4e, f).
NMN protects against pro-inflammatory cytokine-mediated islet dysfunction in isolated mouse islets. a GSIS and (b) LSIS following incubation with NMN (48 h; 100 μmol/l). c GSIS and (d) LSIS following 48 h incubation with IL1β (5 ng/ml) and TNFα (10 ng/ml), with or without NMN (100 μmol/l). e Il1b expression and protein abundance (blot), and (f) Tnfa gene expression following 48 h incubation with IL1β (5 ng/ml) and TNFα (10 ng/ml) (CK). g Il1b gene expression following 48 h incubation with palmitate (100 μmol/l) and glucose (20 mmol/l). h GSIS and (i) LSIS following 48 h incubation with palmitate (100 μmol/l) and glucose (20 mmol/l), with or without NMN (100 μmol/l). Data are expressed as mean±SEM; *p < 0.05, **p < 0.01 and ***p < 0.001 for differences between untreated and cytokine-, palmitate/glucose- or NMN-treated islets; † p < 0.05 and ††† p < 0.001 for effects of co-incubation with NMN. White, untreated; grey (a, b), NMN-treated; grey (c, d), cytokine (CK)+NMN-treated; grey (h, i), palmitate+NMN-treated; black (c, d), cytokine treated only; black (h, i), palmitate-treated only. CON, control
Glucolipotoxicity, which results from elevated circulating levels of glucose and NEFA such as palmitate, can also lead to suppression of islet insulin secretion [26–28], in part through induction of Il1b expression [5, 6, 29]. Consistent with this, exposure of islets to palmitate (100 μmol/l) and glucose (20 mmol/l) in combination for 48 h induced Il1b mRNA (Fig. 4g), and suppressed GSIS and LSIS (Fig. 4h, i). Similarly to the effects seen in cytokine-exposed islets, the effects of palmitate/glucose were reversed by co-incubation with NMN (Fig. 4h, i).
NMN reverses FRD and pro-inflammatory cytokine-mediated changes in expression of genes encoding islet markers
Suppressed GSIS and LSIS in FRD+V mouse islets occurred in parallel with changes in expression of genes encoding key islet markers. Pdx1, which encodes a transcription factor essential for islet beta cell differentiation [30], was suppressed in FRD+V islets compared with CON+V (−21%; p < 0.05) (Fig. 5a). Similarly, expression of the glucose transporter Glut2 (also known as Slc2a2) and glucokinase (Gk, also known as Gck), the latter of which initiates glycolysis following glucose uptake into the islet, and both of which are under the transcriptional control of PDX1 [31], was suppressed by 51% (p < 0.01) and 17% (p < 0.05), respectively (Fig. 5b, c). Similarly to effects on GSIS and LSIS, FRD-mediated changes in gene expression were reversed in FRD-fed mice administered NMN, indicating that NMN improves islet function in part through beneficial changes in expression of several genes essential for glucose sensing and beta cell differentiation. FRD also led to elevated islet mRNA levels of inducible nitric oxide synthase (Inos [also known as Nos2]) (49%; p < 0.05) (Fig. 5d), a target (via nuclear factor κB [NFκB]) of IL1β [32]; inducible nitric oxide synthase induces cellular stress and cell death through production of reactive oxygen species. In addition, mRNA levels of the pro-apoptotic gene Bax (23%; p < 0.05) (Fig. 5e) were elevated in FRD+V mice. FRD-mediated induction of Inos and Bax expression was blocked by NMN (Fig. 5d, e). NMN administration also lowered increased Il1b expression to basal levels in FRD+NMN compared with FRD+V mice (p < 0.001) (Fig. 5f), indicating that a potential anti-inflammatory mechanism mediates the actions of NMN.
Effects of FRD and NMN on islet gene expression. Islets were isolated from CON+V, FRD+V and FRD+NMN mice, and expression of (a) Pdx1, (b) Glut2, (c) Gk, (d) Inos, (e) Bax, (f) Il1b, (g) Tnfa, (h) Sirt1 and (i) Sirt3 was measured by quantitative PCR. Data are expressed as mean±SEM; *p < 0.05, **p < 0.01 and ***p < 0.001 for differences between CON+V and FRD+V islets; † p < 0.05, †† p < 0.01 and ††† p < 0.001 for differences between FRD+V and FRD+NMN islets
Similarly, isolated islets incubated with IL1β/TNFα displayed reduced expression of Pdx1 (72%; p < 0.001), Glut2 (90%; p < 0.01) and Gk (42%; p < 0.05), as well as increased expression of Inos (3.9-fold; p < 0.001) and Bax (twofold; p < 0.001). These changes in gene expression elicited by IL1β/TNFα were reversed by co-incubation with NMN (ESM Fig. 1a–e). Moreover, the IL1β/TNFα-mediated induction of Il1b gene expression and production of the corresponding protein that are described above were also suppressed by NMN (ESM Fig. 1f), supporting the notion of an anti-inflammatory mechanism of NMN action. Expression of two other islet transcription factors, Tfam and Hnf1a, were unchanged by IL1β/TNFα and/or NMN (data not shown). These changes in gene expression in response to pro-inflammatory cytokines and NMN are reminiscent of those observed in islets isolated from FRD-fed mice.
Taken together, these data indicate that NMN improves islet function in FRD-fed mice in association with beneficial changes in expression of genes involved in glucometabolic, anti-inflammatory and apoptotic processes.
NMN-mediated induction of insulin secretion involves sirtuin 1
We next assessed a possible role for sirtuin 1 as a target mediating the actions of NMN in islets. Consistent with a potential role for sirtuin 1 in mediating the effects of NMN, expression of Sirt1 was suppressed by 65% in FRD+V mice (p < 0.05) (Fig. 5h). In addition, FRD+V mice displayed decreased mRNA levels of the mitochondrial sirtuin, Sirt3 (29%; p < 0.05) (Fig. 5i). The role of sirtuin 3 in islet function is not yet known, but it is known to positively regulate mitochondrial ATP production [33–35], which is important for nutrient-stimulated insulin secretion, and to be induced by NAMPT [36, 37]. These effects were reversed by NMN in FRD+NMN mice (Fig. 5h, i). Similarly, IL1β/TNFα treatment led to a 46% (p < 0.01) reduction in Sirt1 mRNA and a 54% (p < 0.001) decrease in Sirt3 mRNA. Co-incubation of NMN with TNFα/IL1β restored Sirt1 and Sirt3 expression to control levels (ESM Fig. 2a, b). Consistent with the notion of a mechanistic role for sirtuin 1 in mediating the effects of NMN, co-incubation of islets with the specific sirtuin 1 inhibitor EX-527 blocked the enhancing effects of NMN alone upon insulin secretion (ESM Fig. 2c). We next investigated whether EX-527 could inhibit the effect of NMN on cytokine-mediated islet dysfunction. The effects of NMN in restoring TNFα/IL1β-mediated suppression of GSIS were partially blocked by EX-527 (45%) (ESM Fig. 2d), suggesting that sirtuin 1 mediates approximately 55% of the protective effects of NMN against cytokine-mediated impairment of GSIS.
Discussion
Previous studies have described a role for pro-inflammatory cytokines in the mediation of beta cell failure [5, 6], a key factor in the progression from insulin resistance to type 2 diabetes. Separately, studies using Nampt +/− mice have highlighted a role for eNAMPT and its reaction product NMN in enhancing pancreatic beta cell function [9]. Here, we report suppressed insulin secretion in response to glucose and leucine in islets cultured with the inflammatory cytokines IL1β and TNFα. Importantly, IL1β/TNFα-mediated suppression of beta cell function was reversed by co-incubation with NMN, indicating that NMN may protect against beta cell failure through an anti-inflammatory mechanism. IL1β can induce its own production through autoinflammatory mechanisms [5, 6]. Consistent with an anti-inflammatory role, co-incubation with NMN reversed IL1β/TNFα-mediated increases in Il1b expression and IL1β abundance.
Increased consumption of diets rich in sugar, particularly fructose, have been linked to the development of type 2 diabetes [21, 22]. We report here that FRD-fed mice displayed dramatically impaired islet insulin secretion compared with control, together with the development of fasting hyperglycaemia and hyperinsulinaemia, as well as islet and adipose tissue inflammation. We therefore further explored the links between NMN and inflammation in vivo in mice fed an FRD. Decreased plasma eNAMPT levels in FRD-fed mice were associated with marked suppression of insulin secretion and elevated islet Il1b expression, both of which were reversed by administration of NMN.
Therefore we propose that adequate tissue delivery and uptake of eNAMPT/NMN are essential for preservation of beta cell function during insulin resistance and that the protective effects of eNAMPT/NMN may occur in part through suppressed expression of genes involved in inflammation. In contrast, during beta cell failure, a decline in eNAMPT/NMN uptake results in loss of protection against chronic inflammation and a consequent rise of IL1β levels, with eventual beta cell death and suppressed insulin secretion.
Further studies will be required to test this hypothesis, with regard to the effects of eNAMPT/NMN on glycaemia and the resolution of insulin resistance, by investigating the impact of NMN administration on insulin and glucose levels during a glucose tolerance test. However, given the positive effects of NMN administration on the resolution of insulin resistance in transgenic models [9, 14], it seems likely that NMN will improve insulin resistance in models of diet-induced insulin resistance. In addition, measurements of plasma NMN are required in models of insulin resistance and type 2 diabetes. NMN measurements were carried out during this study using HPLC; however, these measurements proved to be inconclusive due to variability within groups (data not shown), and a larger study will be required to accurately establish the relationship between eNAMPT, islet dysfunction and NMN.
Interestingly, patients homozygous for either of two single nucleotide polymorphisms in the NAMPT promoter display lower plasma insulin levels, suggesting a connection between NAMPT dysfunction and regulation of insulin secretion in humans [38]. Based on our studies of the effects of NMN on insulin secretion in vitro, it is plausible that elevation of eNAMPT/NMN levels may be part of the mechanism allowing beta cell compensation. We predict that this mechanism maintains insulin secretion by providing protection against chronic inflammation through lowering of islet IL1β levels, which may otherwise be increased. Further studies will be required to assess whether these changes are unique to fructose consumption, but given recent increases in dietary sugar consumption, the results described here are likely to be of relevance in humans. Consistent with the present findings in mice maintained on an FRD for 16 weeks, another study has described increased beta cell apoptosis in rats fed an FRD for 3 weeks [39], but with increased insulin secretion, suggesting that the adverse effects of fructose feeding on islet function is progressive. Further studies will elucidate how these progressively deleterious changes relate to alterations in eNAMPT regulation.
Impaired beta cell function in cytokine-exposed and FRD-fed mice was associated with decreased islet Sirt1 mRNA. Moreover, the sirtuin 1 inhibitor EX-527 [40] blocked the beneficial effects of NMN on nutrient-stimulated insulin secretion in non-cytokine-treated islets and also partially blocked the effects of NMN in cytokine-treated islets. Previous studies have reported that cytokine exposure reduces Sirt1 expression and increases cytotoxicity, Inos expression and nitric oxide production in a beta cell line and in rat islets [32]. In contrast, sirtuin 1 activation or overabundance prevented cytokine-mediated cytotoxicity through a mechanism involving inhibition of NFκB [32]. We propose that NMN induces sirtuin 1 activity, instigating an anti-inflammatory process that culminates in the reversal of increased IL1β levels and enhanced islet function. In turn, lower IL1β levels lead to a reversal of the cytokine-mediated decrease of Sirt1 mRNA levels (Fig. 6). However, since sirtuin 1 inhibition only blocks approximately half of the NMN effect on the islet, NMN may also exert beneficial effects on islets through other NAD+-dependent enzymes, possibly sirtuin 2 to 7. We observed decreased Sirt3 mRNA in FRD-fed mice and IL1β/TNFα-cultured islets. Sirtuin 3 has no previously described function in islets, but has been reported to promote ATP production [33–35], have anti-apoptotic properties and be induced by NMN/NAMPT in other tissues [36]. Further studies are required to elucidate a role for sirtuin 3 and other sirtuins in mediating NMN function in islets.
Schematic of proposed pathways of NMN action and NAMPT suppression in FRD-fed mice. a iNAMPT abundance is increased, whilst plasma eNAMPT levels are decreased in FRD-fed mice. These changes are associated with raised islet IL1β abundance, and suppressed GSIS and LSIS. b Exogenous administration of NMN, a product of the NAMPT reaction, leads to suppression of Il1b mRNA levels, potentially through activation of sirtuin 1 (SIRT1), raised Sirt1 mRNA levels and restoration of GSIS and LSIS
Interestingly, lower plasma levels of eNAMPT/NMN in FRD-fed mice corresponded with increased abundance of iNAMPT in BAT and, to a lesser extent, in WAT. This suggests that lower eNAMPT levels in FRD-fed mice occur through a defect either in eNAMPT secretion, or in the putative post-translational modification [9] that may process iNAMPT for secretion.
In summary, we provide evidence for a novel mechanism that mediates onset of beta cell failure in dietary-induced inflammation via reduced exposure to eNAMPT, leading to increased islet inflammation and impaired beta cell function. Moreover, we show that NMN, an intermediary in the NAMPT reaction, can correct cytokine-induced islet dysfunction, suggesting that modulation of this pathway could be an attractive target for treatment of islet inflammation in type 2 diabetes. Finally, this study provides further evidence of the dangers of consumption of a sugar-rich diet, and may have important implications regarding the pathophysiology of type 2 diabetic patients.
Abbreviations
- BAT:
-
Brown adipose tissue
- CON+V:
-
Control + vehicle
- CON+NMN:
-
Control + nicotinamide mononucleotide
- eNAMPT:
-
Extracellular nicotinamide phosphoribosyltransferase
- FRD:
-
Fructose-rich diet
- FRD+NMN:
-
Fructose-rich diet + nicotinamide mononucleotide
- FRD+V:
-
Fructose-rich diet + vehicle
- GSIS:
-
Glucose-stimulated insulin secretion
- HBSS:
-
Hanks’ buffered salt solution
- iNAMPT:
-
Intracellular nicotinamide phosphoribosyltransferase
- LSIS:
-
Leucine-stimulated insulin secretion
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- NFκB:
-
Nuclear factor κB
- NMN:
-
Nicotinamide mononucleotide
- WAT:
-
White adipose tissue
References
Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79:537–543
Johnson RJ, Segal MS, Sautin Y et al (2007) Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86:899–906
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Henquin JC (2008) Pancreatic beta-cell mass or beta-cell function? That is the question! Diabetes Obes Metab 10:1–4
Donath MY, Boni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA (2010) Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab 21:261–267
Dinarello CA, Donath MY, Mandrup-Poulsen T (2010) Role of IL-1 beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 17:314–321
Chou DHC, Bodycombe NE, Carrinski HA et al (2010) Small-molecule suppressors of cytokine-induced beta-cell apoptosis. ACS Chem Biol 5:729–734
Lawrence MC, Naziruddin B, Levy MF, Jackson A, Mcglynn K (2011) Calcineurin/nuclear factor of activated T cells and MAPK signaling induce TNF-alpha gene expression in pancreatic islet endocrine cells. J Biol Chem 286:1025–1036
Revollo JR, Korner A, Mills KF et al (2007) Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 6:363–375
Imai S (2009) Nicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseases. Curr Pharm Des 15:20–28
Moynihan KA, Grimm AA, Plueger MM et al (2005) Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2:105–117
Bordone L, Motta MC, Picard F et al (2006) Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol 4:e31
Revollo JR, Grimm AA, Imai S (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279:50754–50763
Ramsey KM, Mills KF, Satoh A, Imai S (2008) Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7:78–88
Tappy L, Le KA (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90:23–46
Jurgens H, Haass W, Castaneda TR et al (2005) Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res 13:1146–1156
Rajasekar P, Anuradha CV (2007) Fructose-induced hepatic gluconeogenesis: effect of l-carnitine. Life Sci 80:1176–1183
Nagai Y, Yonemitsu S, Erion DM et al (2009) The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab 9:252–264
Roncal-Jimenez CA, Lanaspa MA, Rivard CJ et al (2011) Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism 60:1259–1270
Rutledge AC, Adeli K (2007) Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65:S13–S23
Bazzano LA, Li TY, Joshipura KJ, Hu FB (2008) Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 31:1311–1317
Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB (2010) Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 33:2477–2483
Stanhope KL, Schwarz JM, Keim NL et al (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119:1322–1334
Teff KL, Grudziak J, Townsend RR et al (2009) Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab 94:1562–1569
Baldwin W, McRae S, Marek G et al (2011) Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 60:1258–1269
Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY (2003) Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 52:726–733
Peyot ML, Pepin E, Lamontagne J et al (2010) Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 59:2178–2187
Prentki M, Nolan CJ (2006) Islet beta cell failure in type 2 diabetes. J Clin Invest 116:1802–1812
Boni-Schnetzler M, Boller S, Debray S et al (2009) Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 150:5218–5229
Oliver-Krasinski JM, Stoffers DA (2008) On the origin of the beta cell. Genes Dev 22:1998–2021
Waeber G, Thompson N, Nicod P, Bonny C (1996) Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol 10:1327–1334
Lee JH, Song MY, Song EK et al (2009) Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes 58:344–351
Ahn BH, Kim HS, Song S et al (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105:14447–14452
Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC (2010) Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 49:304–311
Law IK, Liu L, Xu A et al (2009) Identification and characterization of proteins interacting with SIRT1 and SIRT3: implications in the anti-aging and metabolic effects of sirtuins. Proteomics 9:2444–2456
Yang H, Yang T, Baur JA et al (2007) Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130:1095–1107
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119:2758–2771
Bailey SD, Loredo-Osti JC, Lepage P et al (2006) Common polymorphisms in the promoter of the visfatin gene (PBEF1) influence plasma insulin levels in a French-Canadian population. Diabetes 55:2896–2902
Maiztegui B, Borelli MI, Raschia MA, Del Zotto H, Gagliardino JJ (2009) Islet adaptive changes to fructose-induced insulin resistance: beta-cell mass, glucokinase, glucose metabolism, and insulin secretion. J Endocrinol 200:139–149
Napper AD, Hixon J, McDonagh T et al (2005) Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J Med Chem 48:8045–8054
Acknowledgements
This research was supported by Diabetes UK grants BDA:RD08/0003665, BDA:RD06/0003424 and BDA:RD03/0002725. We thank N.K. Nayuni (William Harvey Research Institute, Queen Mary University of London) for help with HPLC measurements of NMN.
Contribution statement
PWC, JK, MMY, MJH and MCS were responsible for the conception and design, or analysis and interpretation of data. PWC, JK, MJH and MSC contributed to the experimental work. PWC, JK, MMY, MJH and MSC drafted the article or revised it critically. PWC, JK, MMY, MJH and MSC gave final approval of the version to be published.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Quantitative RT-PCR primer sequences (PDF 43 kb)
ESM Fig. 1
Effects of pro-inflammatory cytokines and NMN on mouse islet gene expression. Islets isolated from control mice were incubated with IL1β (5 ng/ml) and TNFα (10 ng/ml) alone or in combination with NMN (100 μmol/l) for 48 h. Gene expression of (a), Pdx1, (b) Glut2, (c) Gk, (d) Inos, (e) Bax, and (f) Il1b were measured by qPCR, (g) IL1β protein levels. Data are expressed as mean±SEM. Statistically significant differences between untreated and cytokine treated islets are indicated by: **p < 0.01, ***p < 0.001. Statistically significant effects of co-incubation with NMN are indicated by: †† p < 0.01, ††† p < 0.001 (PDF 203 kb)
ESM Fig. 2
NMN-mediated induction of insulin secretion involves SIRT1. Gene expression of (a), Sirt1 and (b), Sirt3 in response to 48 h incubation with IL1β (5 ng/ml) and TNFα (10 ng/ml), with or without NMN (100 μmol/l). (c) GSIS in response to 48 h incubation with NMN (100 μmol/l) and EX527 (10 μmol/l) in islets isolated from control mice. (d) GSIS in response to 48 h incubation with IL1β (5 ng/ml) and TNFα (10 ng/ml), NMN (100 μmol/l) and EX527 (10 μmol/l) in islets isolated from control mice. Data are expressed as mean±SEM. Statistically significant differences between untreated and cytokine or NMN treated islets are indicated by: **p < 0.01, ***p < 0.001. Statistically significant effects of NMN in combination with cytokines are indicated by: † p < 0.05, †† p < 0.01, ††† p < 0.001. Statistically significant effects of EX527 are indicated by: ++ p < 0.01 (PDF 203 kb)
Rights and permissions
About this article
Cite this article
Caton, P.W., Kieswich, J., Yaqoob, M.M. et al. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia 54, 3083–3092 (2011). https://doi.org/10.1007/s00125-011-2288-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2288-0