Abstract
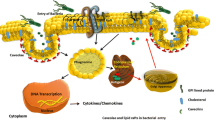
Distinct domains in the cell membrane, termed rafts, emerge as central for the infection of mammalian cells by many pathogens. Rafts consist of sphingolipids and cholesterol that interact strongly, and thus spontaneously separate from other phospholipids in the cell membrane. Recent studies suggest that at least some pathogens activate the acid sphingomyelinase that releases ceramide in membrane rafts. The generation of ceramide transforms small rafts into a signaling unit and results in the fusion of small rafts to large platforms. Membrane rafts and ceramide-enriched membrane platforms have been shown to mediate internalization of bacteria, viruses and parasites into the host cell, to initiate apoptosis of the host cell upon infection and to regulate the release of cytokines from infected mammalian cells. Furthermore, rafts and ceramide have been implicated in the intracellular trafficking of phagosomes and in the budding of viruses from infected cells. The molecular function of rafts and ceramide-enriched membrane platforms seems to be the re-organization of receptor and intracellular signaling molecules in the cell membrane permitting the interaction of the pathogen with the cell. This suggests that rafts and ceramide-enriched membrane platforms function as central structures involved in the infection of mammalian cells by pathogens and as targets for the development of anti-infective drugs.


Similar content being viewed by others
References
Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Brown DA, London E (1998) Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164:103–114
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Anderson RGW, Jacobson K (2002) A role for lipid shells in targeting proteins to caveolae, rafts and other lipid domains. Science 296:1821–1825
Kolesnick RN, Goni FM, Alonso A (2000) Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol 184:285–300
Grassmé H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E (2001) CD95 signaling via ceramide rich membrane rafts. J Biol Chem 276:20589–20596
Grassmé H, Jendrossek V, Bock J, Riehle A, Gulbins E (2002) Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol 168:298–307
Grassmé H, Jendrossek V, Riehle A, von Kürthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E (2003) Host defense against P. aeruginosa requires ceramide-rich membrane rafts. Nat Med 9:322–330
Gulbins E, Kolesnick RN (2003) Raft ceramide in molecular medicine. Oncogene 22:7070–7077
Cremesti A, Paris F, Grassmé H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R (2001) Ceramide enables Fas to cap and kill. J Biol Chem 276:23954–23961
Bock J, Gulbins E (2002) The transmembranous domain of CD40 determines CD40 partitioning into lipid rafts. FEBS Lett 534:169–174
Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R (2003) Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300:1155–1159
Zhang Y, Mattjus P, Schmid PC, Dong Z, Zhong S, Ma WY, Brown RE, Bode AM, Schmid HH, Dong Z (2001) Involvement of the acid sphingomyelinase pathway in UVA-induced apoptosis. J Biol Chem 276:11775–11782
Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman EH, Kolesnick RN, Tilly JL (2000) Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1- phosphate therapy. Nat Med 6:1109–1114
Fanzo JC, Lynch MP, Phee H, Hyer M, Cremesti A, Grassmé H, Norris JS, Coggeshall KM, Rueda BR, Pernis AB, Kolesnick R, Gulbins E (2003) CD95 rapidly clusters in cells of diverse origins. Cancer Biol Ther 2:392–395
Hueber AO, Bernard AM, Herincs Z, Couzinet A, He HT (2002) An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep 3:190–196
Holopainen JM, Subramanian M, Kinnunen PK (1998) Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidycholine/sphingomyelin membrane. Biochemistry 37:17562–17570
Nurminen TA, Holopainen JM, Zhao H, Kinnunen PK (2002) Observation of topical catalysis by sphingomyelinase coupled to microspheres. J Am Chem Soc 124:12129–12134
Huang HW, Goldberg EM, Zidovetzki R (1999) Ceramides modulate protein kinase C activity and perturb the structure of phosphatidylcholine/phosphatidylserine bilayers. Biophys J 77:1489–1497
Veiga MP, Arrondon JL, Goni FM, Alonso A (1999) Ceramides in phospholipid membranes: effects on bilayer stability and transition to nonlamellar phases. Biophys J 76:342–350
Grotenhuis E ten, Demel RA, Ponec M, Boer DR, van Miltenburg JC, Bouwstra JA (1996) Phase behavior of stratum corneum lipids in mixed Langmuir-Blodgett monolayers. Biophys J 71:1389–1399
Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobsson K, Gratton E (2001) Lipid rafts reconstituted in model membranes. Biophys J 80:1417–1428
Grassmé H, Gulbins E, Brenner B, Ferlinz K, Sandhoff K, Harzer K, Lang F, Meyer TF (1997) Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 91:605–615
Hauck CR, Grassmé H, Bock J, Jendrossek V, Ferlinz K, Meyer TF, Gulbins E (2000) Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of N. gonorrhoeae. FEBS Lett 478:260–266
Esen M, Schreiner B, Jendrossek V, Lang F, Fassbender K, Grassmé H, Gulbins E (2001) Mechanisms of Staphylococcus aureus induced apoptosis of human endothelial cells. Apoptosis 6:441–445
Shin JS, Gao Z, Abraham SN (2000) Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785–787
Baorto DM, Gao Z, Malaviya R, Dustin M, van der Merwe A, Lublin DM, Abraham SN (1997) Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389:636–639
Zobiack N, Rescher U, Laarmann S, Michgehl S, Schmidt MA, Gerke U (2002) Cell-surface attachment of pedestal-forming enteropathogenic E. coli induces a clustering of raft components and a recruitment of annexin. J Cell Sci 115:91–98
Cannon CL, Kowalski MP, Stopak KS, Pier GB (2003) Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am J Respir Cell Mol Biol 229:188–197
Anes E, Kühnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G (2003) Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol 5:793–802
Bini L, Pacini S, Liberatori S, Valensin S, Pellegrini M, Raggiaschi R, Pallini V, Baldar CT (2003) Extensive temporally regulated reorganization of the lipid raft proteome following T-cell antigen receptor triggering. Biochem J 369:301–309
Scheel-Toellner D, Wang K, Sing R, Majeed S, Raza K, Curnow SJ, Salmon M, Lord JM (2002) The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun 297:876–879
Grassmé H, Cremesti A, Kolesnick R, Gulbins E (2003) Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene 22:5457–5470
Pier GB, Grout M, Zaidi TS, Olsen JC, Johnson LG, Yankaskas JR, Goldberg JB (1996) Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 271:64–67
Grassmé H, Kirschnek S, Riethmueller J, Riehle A, von Kürthy G, Lang F, Weller M, Gulbins E (2000) Host defense to Pseudomonas aeruginosa requires CD95/CD95 ligand interaction on epithelial cells. Science 290:527–530
Simons K, Ehehalt R (2002) Cholesterol, lipid rafts and disease. J Clin Invest 110:597–603
Jan JT, Chatterjee S, Griffin DE (2000) Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J Virol 74:6425–6432
Hanada K, Palacpac NM, Magistrado PA, Kurokawa K, Rai G, Sakata D, Hara T, Horii T, Nishijima M, Mitamura T (2002) Plasmodium falciparum phospholipase C hydrolyzing sphingomyelin and lysocholinephospholipids is a possible target for malaria chemotherapy. J Exp Med 195:23–34
Graham DR, Chertova E, Hilburn JM, Arthur LO, Hildreth JE (2003) Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J Virol 77:8237–8248
Walmsley AR, Zeng F, Hooper NM (2003) The N-terminal region of the prion protein ectodomain contains a lipid rafts targeting determinant. J Biol Chem 278:37241–37248
Tarboulos AM, Scott M, Semenov A, Avraham D, Laszlo L, Prusiner SB (1995). Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J Cell Biol 129:121–132
Baron GS, Wehrly K., Dorward DW, Chesebro B, Caughey B (2002) Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res [PrP(Sc)] into contiguous membranes. EMBO J 21:1031–1040
Acknowledgements
Studies described in this review were supported by DFG grant Gu 335/10-2/3 to E.G. and the IFORES program to H.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gulbins, E., Dreschers, S., Wilker, B. et al. Ceramide, membrane rafts and infections. J Mol Med 82, 357–363 (2004). https://doi.org/10.1007/s00109-004-0539-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-004-0539-y