Abstract
Dipeptidyl peptidase-4 (DPP-4) inhibitors collectively comprise a presently unique form of disease management for persons with type 2 diabetes mellitus. The aim of this review is to compare the clinical pharmacokinetics of available DPP-4 inhibitors (alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin) for the purpose of identifying potential selection preferences according to individual patient variables and co-morbidities.
DPP-4 inhibitors are readily absorbed orally. Following oral ingestion, absorption occurs mainly in the small intestine, with median times to maximum (peak) plasma concentration ranging from 1 to 3 hours. The fraction of each dose absorbed ranges from approximately 30% with linagliptin to 75–87% for all others. Numerical differences in maximum (peak) plasma drug concentrations and areas under the plasma concentration-time curve among the DPP-4 inhibitors vary by an order of magnitude. However, functional capacity measured in terms of glucose-lowering ability remains comparable among all available DPP-4 inhibitors.
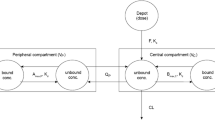
Distribution of DPP-4 inhibitors is strongly influenced by both lipophilicity and protein binding. Apparent volumes of distribution (Vd) for most agents range from 70 to 300 L. Linagliptin exhibits a Vd of more than 1000 L, indicating widespread distribution into tissues. Binding to target proteins in plasma and peripheral tissues exerts a major influence upon broadening linagliptin distribution.
DPP-4 inhibitor metabolism is widely variable, with reported terminal half-lives ranging from approximately 3 to more than 200 hours. Complex relationships between rates of receptor binding and dissociation appear to strongly influence the durations of action of those DPP-4 inhibitors with comparatively shorter half-lives. Durations of activity often are not reflective of clearance and, with the exception of vildagliptin which may be administered either once daily in the evening or twice daily, these medications are effective when used with a once-daily dosing schedule.
Saxagliptin and, to a lesser extent, sitagliptin are largely metabolized by hepatic cytochrome P450 (CYP) 3A4 and 3A5 isoforms. With the exception of the primary hydroxylated metabolite of saxagliptin, which is 2-fold less potent than its parent molecule, metabolic products of hepatic biotransformation are minimally active and none appreciably contribute to either the therapeutic or the toxic effects of DPP-4 inhibitors.
No DPP-4 inhibitor has been shown to inhibit or to induce hepatic CYP-mediated drug metabolism. Accordingly, the number of clinically significant drug-drug interactions associated with these agents is minimal, with only saxagliptin necessitating dose adjustment if administered concurrently with medications that strongly inhibit CYP3A4.
Linagliptin undergoes enterohepatic cycling with a large majority (85%) of the absorbed dose eliminated in faeces via biliary excretion. Other DPP-4 inhibitors predominantly undergo renal excretion, with 60–85% of each dose eliminated as unchanged parent compound in the urine.
Systematic reviews of clinical trials suggest that the overall efficacy of DPP-4 inhibitors in patients with type 2 diabetes generally is similar. Apart from these generalizations, pharmacokinetic distinctions that potentially influence product selection are tentative. When considered in total, data reviewed in this report suggest that the best overall balance between potency and the clinical pharmacokinetic characteristics of distribution, metabolism and elimination may be observed with linagliptin followed closely by vildagliptin, saxagliptin, sitagliptin and alogliptin.








Similar content being viewed by others
References
Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest 1967; 46: 1954–62
McIntyre N, Holdsworth CD, Turner DS. Intestinal factors in the control of insulin secretion. J Clin Endocrinol Metab 1965; 25: 1317–24
Elrick H, Stimmler L, Hlad Jr CJ, et al. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 1964; 24: 1076–82
Dupré J. An intestinal hormone affecting glucose disposal in man. Lancet 1964; 2: 672–3
Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986; 63: 492–8
Nauck M, Stöckmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29: 46–52
Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 2004; 287: E199–206
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 2007; 87: 1409–39
Bagger JI, Knop FK, Lund A, et al. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: 737–45
Holst JJ, Krarup T, Knop FK, et al. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 2011; 34 Suppl. 2: S251–7
Hare KJ, Vilsbøll T, Asmar M, et al. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 2010; 59: 1765–70
Flint A, Raben A, Astrup A, et al. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–20
Waget A, Cabou C, Masseboeuf M, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology 2011; 152: 3018–29
Marchetti C, Di Carlo A, Facchiano F, et al. High mobility group box 1 is a novel substrate of dipeptidyl peptidase-IV. Diabetologia 2012; 55: 236–44
Duttaroy A, Voelker F, Merriam K, et al. The DPP-4 inhibitor vildagliptin increases pancreatic beta cell mass in neonatal rats. Eur J Pharmacol 2011; 650: 703–7
Foley JE, Bunck MC, Möller-Goede DL, et al. Beta cell function following 1 year vildagliptin or placebo treatment and after 12 weeks washout without in drug-naïve patients with type 2 diabetes and mild hyperglycaemia: a randomised controlled trial. Diabetologia 2011; 54: 1985–91
Nauck MA, Meininger G, Sheng D, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9: 194–205
Pospislilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates β-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 2003; 52: 741–50
Seck T, Nauck M, Sheng D, et al. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. Int J Clin Pract 2010; 64: 562–76
van Genugten RE, van Raalte DH, Daimant M. Dipeptidyl peptidase-4 inhibitors and preservation of pancreatic islet-cell function: a critical appraisal of the evidence. Diabetes Obes Metab 2012; 14: 101–11
Zhang X, Wang Z, Huang Y, et al. Effects of chronic administration of alogliptin on the development of diabetes and β-cell function in high fat diet/streptozotocin diabetic mice. Diabetes Obes Metab 2011; 13: 337–47
Chen B, Moore A, Escobedo LVS, et al. Sitagliptin lowers glucagon and improves tolerance in prediabetic SHROB rats. Exp Biol Med 2011; 236: 309–14
Migoya EM, Bergeron R, Miller JL, et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin Pharmacol Ther 2010; 88: 801–8
Dobrian AD, Ma Q, Lindsay JW, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab 2011; 300: E410–21
Shirakawa J, Fujii H, Ohnuma K, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes 2011; 60: 1246–57
Miura K, Kitahara Y, Yamagishi S. Combination therapy with nateglinide and vildagliptin improves postprandial metabolic derangements in Zucker fatty rats. Horm Metab Res 2010; 42: 731–5
Matikainen N, Mänttari S, Schweizer A, et al. Vildagliptin therapy reduces intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia 2006; 49: 2047–57
Mason RP, Jacob RF, Kubant R, et al. Effect of enhanced glycemic control with saxagliptin on endothelial nitric oxide release and CD40 levels in obese rats. J Atheroscler Thromb 2011; 18: 774–83
Lamanna C, Monami M, Bartoli N, et al. Dipeptidyl peptidase-4 inhibitors and cardiovascular events: a protective effect? [abstract]. European Association for the Study of Diabetes (EASD) 47th Annual Meeting. Lisbon, Portugal: September 20, 2011 [online]. Available from URL: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=2f88f1ba-1c6c-4863-9521-2def7b443f46&cKey=48d7640c-6588-4847-916d-fbce529f98eb&mKey=%7BBAFB2746-B0DD-4110-8588-E385FAF957B7%7D [Accessed 2011 Nov 8]
Kühn-Wache K, Bär JW, Hoffmann T, et al. Selective inhibition of dipeptidyl peptidase 4 by targeting a substrate-specific secondary binding site. Biol Chem 2011; 392: 223–31
Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess 2010; 14: 1–248
Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 2011; 34 Suppl. 2: S279–84
Monami M, Marchionni N, Mannuchi E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. Eur J Endocrinol 2009; 160: 909–17
Monami M, Cremasco F, Lamanna C, et al. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized and clinical trials. Exp Diabetes Res 2011; 2011: 215674
Faknoury WK, Lereun C, Wright D. A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology 2010; 86: 44–57
Richter B, Bandeira-Echtler E, Bergerhoff K, et al. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008; (2): CD006739
Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: acomparative review. Diabetes Obes Metab 2011; 13: 7–18
Esposito K, Cozzolino D, Bellastella G, et al. Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab 2011; 13: 594–603
Monami M, Cremasco F, Lamanna C, et al. Predictors of response to dipeptidyl peptidase-4 inhibitors: evidence from randomized clinical trials. Diabetes Metab Res Rev 2011; 27: 362–72
Januvia® tablets [package insert]. Whitehouse Station (NJ): Merck & Co. Inc., 2010
Onglyza™ tablets [package insert]. Princeton (NJ): Bristol-Myers Squibb Co., 2009
Tradjenta™ tablets [package insert]. Ridgefield (CT): Boehringer Ingelheim Pharmaceuticals Inc., 2011
European Medicines Agency. Galvus: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf [Accessed 2012 Feb 28]
European Medicines Agency. Januvia: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000722/WC500039054.pdf [Accessed 2011 Oct 13]
European Medicines Agency. Onglyza: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001039/WC500044316.pdf [Accessed 2011 Oct 13]
European Medicines Agency. Trajenta: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002110/WC500115745.pdf [Accessed 2011 Nov 7]
Ogata S, Misumi Y, Ikehara Y. Primary structure of rat liver dipeptidyl peptidase IV deduced from its cDNA and identification of the NH2-terminal signal sequence as the membrane-anchoring domain. J Biol Chem 1989; 264: 3596–501
Vilsbøll T, Agersø H, Krarup T, et al. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 2003; 88: 220–4
Green BD, Flatt PR, Bailey CJ. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc Dis Res 2006; 3: 159–65
Lambeir A-M, Scharpé S, DeMeester I. DPP4 inhibitors for diabetes: what’s next? Biochem Pharmacol 2008; 76: 1637–43
Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs 2011; 71: 1441–67
Lambeir A-M, Durinx C, Scharpé S, et al. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 2003; 40: 209–94
Oefner C, D’Arcy A, MacSweeney A, et al. High-resolution structure of human apo dipeptidyl peptidase IV/CD26 and its complex with 1-[([2-[(5-iodopyridin-2-yl)amino]-ethyl]amino)-acetyl]-2-cyano-(S)-pyrollidine. Acta Crystallogr D Biol Crystallogr 2003; 59: 1206–12
Gerich J. DPP-4 inhibitors: what may be the clinical differentiators? Diabetes Res Clin Pract 2010; 90: 131–40
Gupta R, Walunj SS, Tokala RK, et al. Emerging drug candidates of dipeptidyl peptidase IV (DPP IV) inhibitor class for the treatment of type 2 diabetes. Curr Drug Targets 2009; 10: 71–87
Kirby M, Yu DMT, O’Connor SP, et al. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci 2010; 118: 31–41
Feng J, Zhang Z, Wallace MB, et al. Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J Med Chem 2007; 50: 2297–300
Thomas L, Eckhardt M, Langkopf E, et al. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther 2008; 325: 175–82
Eckhardt M, Langkopf E, Mark M, et al. 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J Med Chem 2007; 50: 6450–3
Augeri DJ, Robl JA, Betebenner DA, et al. Discovery and preclinical profile of saxagliptin (BMS-477118): a highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 2005; 48: 5025–7
Kim D, Wang L, Beconi M, et al. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-di-hydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 2005; 48: 141–51
Kim YB, Kopcho LM, Kirby MS, et al. Mechanism of Gly-Pro-pNA cleavage catalyzed by dipeptidyl peptidase-IV and its inhibition by saxagliptin (BMS-477118). Arch Biochem Biophys 2006; 445: 9–18
Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006; 91: 4612–9
Herman GA, Stein PP, Thornberry NA, et al. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther 2007; 81: 761–7
Villhauer EB, Brinkman JA, Naderi GB, et al. 1-[[(3-hydroxy-1-adamantyl) amino)acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J Med Chem 2003; 16: 2774–89
Brandt I, Joossens J, Chen X, et al. Inhibition of dipeptidyl-peptidase IV catalyzed peptide truncation by vildagliptin ((2S)-[(3-hydroxyadamantan-1-yl) amino]acetyl-pyrrolidine-2-carbonitrile). Biochem Pharmacol 2005; 70:134–43
Coughlan A, McCarty DJ, Jorgensen LN, et al. The epidemic of NIDDM in Asian and Pacific Island populations: prevalence and risk factors. Horm Metab Res 1997; 29: 323–31
Kawamori R. Diabetes trends in Japan. Diabetes Metab Res Rev 2002; 18 Suppl. 3: S9–13
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and prevention strategies [published erratum appears in Lancet 2004; 363 (9412): 902]. Lancet 2004; 363: 902
Christopher R, Covington P, Davenport M, et al. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl-4 inhibitor alogliptin in healthy male subjects. Clin Ther 2008; 30: 513–27
Christopher R, Karim A. Clinical pharmacology of alogliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of type 2 diabetes. Expert Rev Clin Pharmacol 2009; 2: 589–600
Hirayama M, Matsuno K, Fujita T, et al. Pharmacokinetics, pharmaco-dynamics, and tolerability of single and multiple doses of the dipeptidyl peptidase-4 inhibitor alogliptin in Japanese healthy male subjects [abstract no. 521]. Diabetes 2008; 57 Suppl. 1: A155
Scott LJ. Alogliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs 2010; 70: 2051–72
Covington P, Christopher R, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: a randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther 2008; 30: 499–512
Sarashina A, Sesoko S, Nakashima M, et al. Linagliptin, a dipeptidyl peptidase-4 inhibitor in development for the treatment of type 2 diabetes mellitus: a phase I, randomized, double-blind, placebo-controlled trial of single and multiple escalating doses in healthy adult male Japanese subjects. Clin Ther 2010; 32: 1188–204
Blech S, Ludwig-Schwellinger E, Gräfe-Mody EU, et al. The metabolism and disposition of the oral dipeptidyl peptidase-4 inhibitor, linagliptin, in humans. Drug Metab Dispos 2010; 38: 667–78
Retlich S, Duval V, Ring A, et al. Pharmacokinetics and pharmacodynamics of single rising intravenous doses (0.5mg-10mg) and determination of absolute bioavailability of the dipeptidyl peptidase-4 inhibitor linagliptin (BI 1356) in healthy male subjects. Clin Pharmacokinet 2010; 49: 829–40
Forst T, Uhlig-Laske B, Ring A, et al. The oral DPP-4 inhibitor linagliptin significantly lowers HbA1c after 4 weeks of treatment in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2011; 13: 542–50
Heise T, Graefe-Mody EU, Hüttner S, et al. Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab 2009; 11:786–94
Horie Y, Kanada S, Watada H, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor linagliptin: a 4-week multicenter, randomized, double-blind, placebo-controlled phase IIa study in Japanese type 2 diabetes patients. Clin Ther 2011; 33: 973–89
Boulton DW, Li L, Frevert EU, et al. Influence of renal or hepatic impairment on the pharmacokinetics of saxagliptin. Clin Pharmacokinet 2011; 50:253–65
Patel CG, Zhang J, Li L, et al. Effect of a high-fat meal on the pharmacokinetics of saxagliptin in healthy subjects. J Clin Pharmacol 2010; 50: 1211–6
Boulton DW, Smith CH, Li L, et al. Bioequivalence of saxagliptin/metformin extended-release (XR) fixed-dose combination tablets and single-component saxagliptin and single-component saxagliptin and metformin XR tablets in healthy subjects. Clin Drug Investig 2011; 31: 619–30
Vincent SH, Reed JR, Bergman AJ, et al. Metabolism and excretion of the dipeptidyl peptidase 4 inhibitor [14C]sitagliptin in humans. Drug Metab Dispos 2007; 35: 533–8
Bergman A, Ebel D, Liu F, et al. Absolute bioavailability of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, in healthy volunteers. Biopharm Drug Dispos 2007; 28: 315–22
Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78: 75–88
Bergman AJ, Stevens C, Zhao YY, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl pepti-dase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther 2006; 28: 55–72
Herman GA, Mistry GC, Yi B, et al. Evaluation of pharmacokinetic parameters and dipeptidyl peptidase-4 inhibition following single doses of sitagliptin in healthy, young Japanese males. Br J Clin Pharmacol 2011; 71: 429–36
He Y-L, Sadler BM, Sabo R, et al. The absolute oral bioavailability and population-based pharmacokinetic modelling of a novel dipeptidylpeptidase-IV inhibitor, vildagliptin, in healthy volunteers. Clin Pharmacokinet 2007; 46: 787–802
He H, Tran P, Yin H, et al. Absorption, metabolism, and excretion of [14C]vildagliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans. Drug Metab Dispos 2009; 37: 536–44
He Y-L, Valencia J, Zhang Y, et al. Hormonal and metabolic effects of morning or evening dosing of the dipeptidyl peptidase IV inhibitor vildagliptin in patients with type 2 diabetes. Br J Clin Pharmacol 2010; 70: 34–42
He Y-L, Yamaguchi M, Ito H, et al. Pharmacokinetics and pharmacodynamics of vildagliptin in Japanese patients with type 2 diabetes. Int J Clin Pharmacol Ther 2010; 48: 582–95
Karim A, Covington P, Christopher R, et al. Pharmacokinetics of alogliptin when administered with food, metformin, or cimetidine: a two-phase crossover study in healthy subjects. Int J Clin Pharmacol Ther 2010; 48: 46–58
Sunkara G, Sabo R, Wang Y, et al. Dose proportionality and the effect of food on vildagliptin, a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers. J Clin Pharmacol 2007; 47: 1152–8
Greischel A, Binder R, Baierl J. The dipeptidyl peptidase-4 inhibitor linagliptin exhibits time- and dose-dependent localization in kidney, liver, and intestine after intravenous dosing: results from high resolution auto-radiography in rats. Drug Metab Dispos 2010; 38: 1443–8
Fuchs H, Binder R, Greischel A. Tissue distribution of the novel DPP-4 inhibitor BI 1356 is dominated by saturable binding to its target in rats. Biopharm Drug Dispos 2009; 30: 229–40
Retlich S, Withopf B, Breischel A, et al. Binding to dipeptidyl peptidase-4 determines the disposition of linagliptin (BI 1356): investigations in DPP-4 deficient and wildtype rats. Biopharm Drug Dispos 2009; 30: 422–36
Retlich S, Duval V, Graefe-Mody U, et al. Impact of target-mediated drug disposition on linagliptin pharmacokinetics and DPP4 inhibition in type 2 diabetic patients. J Clin Pharmacol 2010; 50: 873–85
Deacon CF, Holst JJ. Saxagliptin: a new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther 2009; 26: 488–99
He Y-L, Serra D, Wang Y, et al. Pharmacokinetics and pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin Pharmacokinet 2007; 46: 577–88
Chu X-Y, Bleasby K, Yabut J, et al. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther 2007; 321: 673–83
Karim A, Fleck P, Hetman L, et al. Single-dose pharmacokinetics of the dipeptidyl peptidase-4 inhibitor alogliptin in subjects with renal impairment [abstract no. 538-P]. Diabetes 2008; 57 Suppl. 1: A160
Graefe-Mody U, Friedrich C, Port A, et al. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin. Diabetes Obes Metab 2011; 13: 939–46
Bergman AJ, Smith W, Cote J, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care 2007; 30: 1862–4
Chan JCN, Scott R, Arjona Ferreira AC, et al. Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab 2008; 10: 549–55
Migoya EM, Stevens CH, Bergman AJ, et al. Effect of moderate hepatic insufficiency on the pharmacokinetics of sitagliptin. Can J Clin Pharmacol 2009; 16: e165–70
Herman GA, Bergman A, Liu F, et al. Pharmacokinetics and pharmacodynamic effects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol 2006; 46: 876–86
He Y-L, Sabo R, Campestrini J, et al. The effect of age, gender, and body mass index on the pharmacokinetics and pharmacodynamics of vildagliptin in healthy volunteers. Br J Clin Pharmacol 2007; 65: 338–46
He YL, Flannery B, Wang Y, et al. The influence of renal impairment on the pharmacokinetics of vildagliptin [abstract no. PIII-86]. Clin Pharmacol Ther 2007; 81 Suppl. 1: S113
He Y-L, Sabo R, Campestrini J, et al. The influence of hepatic impairment on the pharmacokinetics of the dipeptidyl peptidase IV (DPP-4) inhibitor vildagliptin. Eur J Clin Pharmacol 2007; 63: 677–86
Lukashevich V, Schweizer A, Shao Q, et al. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab 2011; 13: 947–54
Banerji MA, Purkayastha D, Francis BH. Safety and tolerability of vildagliptin vs. thiazolidinedione as add-on to metformin in type 2 diabetic patients with and without mild renal impairment: a retrospective analysis of the GALIANT study. Diabetes Res Clin Pract 2010; 90: 182–90
Ito M, Abe M, Okada K, et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Endocr J 2011; 58: 979–87
Ligueros-Saylan M, Foley JE, Schweizer A, et al. An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of phase II and III clinical trials. Diabetes Obes Metab 2010; 12: 495–509
Nowicki M, Rychlik I, Haller H, et al. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract 2011; 65: 1230–9
Krishnarajah G, Dezil C, Tran M, et al. Evaluation of sitagliptin dosing among renal impaired type 2 diabetes patients [abstract no. PDB70]. Value Health 2010; 13: A67
McFarland MS, Cross LB, Gross B, et al. Drug use evaluation of sitagliptin dosing by pharmacist versus nonpharmacist clinicians in an internal medicine department of a private physician-owned multispecialty clinic. J Manag Care Pharm 2009; 15: 563–7
Quasem M, Liu Y. Acute interstitial nephritis associated with sitagliptin use [abstract no. 257]. Am J Kidney Dis 2011; 57(4): A81
Lestner JM, Baburaj R, Edwards CMB. Renal impairment with sitagliptin: is there a need for active monitoring of potential renal toxicity? Br J Hosp Med 2011; 72: 412–3
Kao DP, Kohrt HE, Kugler J. Renal failure and rhabdomyolysis associated with sitagliptin and simvastatin use. Diabet Med 2008; 25: 1229–30
Patel CG, Li L, Girgis S, et al. Two-way pharmacokinetic interaction studies between saxagliptin and cytochrome P450 substrates or inhibitors: simvastatin, diltiazem extended-release, and ketoconazole. Clin Pharmacol Adv Appl 2011; 2: 13–25
Dhillon S, Weber J. Saxagliptin. Drugs 2009; 69: 2103–14
DiGregorio RV, Pasikova Y. Rhabdomyolysis caused by a potential sitagliptin-lovastatin interaction. Pharmacotherapy 2009; 29: 352–6
Bhome R, Penn H. Rhabdomyolysis precipitated by a sitagliptin-atorvastatin drug interaction. Diabet Med 2012; 29: 693–4
Pratley RE. Alogliptin: a new, highly selective dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Expert Opin Pharmacother 2009; 10: 503–12
Karim A, Laurent A, Munsaka M, et al. Coadministration of pioglitazone or glyburide and alogliptin: pharmacokinetic drug interaction assessment in healthy participants. J Clin Pharmacol 2009; 49: 1210–9
Friedrich C, Ring A, Brand T, et al. Evaluation of the pharmacokinetic interaction after multiple oral doses of linagliptin and digoxin in healthy volunteers. Eur J Drug Metab Pharmacokinet 2011; 36: 17–24
Friedrich C, Port A, Ring A, et al. Effect of multiple oral doses of linagliptin on the steady-state pharmacokinetics of a combination oral contraceptive in healthy female adults: an open-label, two-period, fixed-sequence, multiple-dose study. Clin Drug Investig 2011; 31: 643–53
Graefe-Mody_U, Rose P, Ring A, et al. Assessment of the pharmacokinetic interaction between the novel DPP-4 inhibitor linagliptin and a sulfonylurea, glyburide, in healthy subjects. Drug Metab Pharmacokinet 2011; 26: 123–9
Graefe-Mody EU, Jungnik A, Ring A, et al. Evaluation of the pharmacokinetic interaction between the dipeptidyl peptidase-4 inhibitor linagliptin and pioglitazone in healthy volunteers. Int J Clin Pharmacol Ther 2010; 48: 652–61
Upreti VV, Boulton DW, Li L, et al. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of saxagliptin, a dipeptidyl peptidase-4 inhibitor, in healthy subjects. Br J Clin Pharmacol 2011; 72: 92–102
Krishna R, Bergman A, Larson P, et al. Effect of a single cyclosporine dose on the single-dose pharmacokinetics of sitagliptin (MK-0431), a dipeptidyl pepitdase-4 inhibitor, in healthy male subjects. J Clin Pharmacol 2007; 47: 165–74
Kasichayanula S, Liu X, Shyu WC, et al. Lack of pharmacokinetic interaction between dapagliflozin, a novel sodium-glucose transporter 2 inhibitor, and metformin, pioglitazone, glimepiride or sitagliptin in healthy subjects. Dia-betes Obes Metab 2011; 13: 47–54
Mistry GC, Bergman AJ, Zheng W, et al. Sitagliptin, an dipeptidyl peptidase-4 inhibitor, does not alter the pharmacokinetics of the sulphonylurea, glyburide, in healthy subjects. Br J Clin Pharmacol 2008; 66: 36–42
Mistry GC, Bergman AJ, Luo W-L, et al. Multiple-dose administration of sitagliptin, a dipeptidyl peptidase-4 inhibitor, does not alter the single-dose pharmacokinetics of rosiglitazone in healthy subjects. J Clin Pharmacol 2007; 47: 159–64
Bergman AJ, Cote J, Maes A, et al. Effect of sitagliptin on the pharmacokinetics of simvastatin. J Clin Pharmacol 2009; 49: 483–8
Odegaard D, Lane J, Stevens RB. Sitagliptin use in kidney transplant recipients: effect on antidepressant levels [abstract]. Diabetes 2009; 58 Suppl.: A1
Wright DH, Herman GA, Maes A, et al. Multiple doses of sitagliptin, a selective DPP-4 inhibitor, do not meaningfully alter pharmacokinetics and pharmacodynamics of warfarin. J Clin Pharmacol 2009; 49: 1157–67
He Y-L, Ligueros-Saylan M, Sunkara G, et al. Vildagliptin, a novel dipeptidyl peptidase IV inhibitor, has not pharmacokinetic interactions with the anti-hypertensive agents amlodipine, valsartan, and ramipril in healthy subjects. J Clin Pharmacol 2008; 48: 85–95
Scheen AJ, Charpentier G, Östgren CJ, et al. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab Res Rev 2010; 26: 540–9
Signorovitch JE, Wu EQ, Swallow E, et al. Comparative efficacy of vildagliptin and sitagliptin in Japanese patients with type 2 diabetes mellitus: a matching-adjusted indirect comparison of randomized trials. Clin Drug Investig 2011; 31: 665–74
Acknowledgements
Dr Golightly and Ms Drayna attest that they have no potential financial conflicts of interest to disclose. Dr McDermott formerly was a member of the speakers bureau of Merck & Co. and has received honoraria for speaking through speakers’ bureaus for Eli Lilly, Novo Nordisk, Amylin, Sanofi Aventis, Takeda and Merck & Co., and is on the Advisory Board of Sanofi Aventis; he previously was a recipient of honoraria granted for providing specialized educational programmes in clinical endocrinology.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found online at http://dx.doi.org/10.1007/s40262-012-0019-4.
Rights and permissions
About this article
Cite this article
Golightly, L.K., Drayna, C.C. & McDermott, M.T. Comparative Clinical Pharmacokinetics of Dipeptidyl Peptidase-4 Inhibitors. Clin Pharmacokinet 51, 501–514 (2012). https://doi.org/10.1007/BF03261927
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261927