Abstract
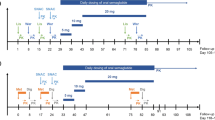
The aim of this study was to investigate whether multiple doses of the oral and highly selective dipeptidyl peptidase-4 inhibitor linagliptin affect the steady-state pharmacokinetics of the P-glycoprotein substrate digoxin. This single-center, open-label, two-period cross-over study involved healthy subjects (n = 20), randomized to treatment sequence AB or BA, where A comprised 0.25 mg digoxin qd for 5 days, then 0.25 mg digoxin qd plus 5 mg linagliptin qd for 6 days, and B comprised 0.25 mg digoxin qd for 11 days. A treatment-free period (≥35 days for AB and 14 days for BA) separated each treatment in both sequences. There were no clinically significant changes in steady-state pharmacokinetic parameters of digoxin when it was co-administered with linagliptin. The ratio of the adjusted-by-treatment geometric mean ratios and associated 90% confidence intervals for the AUCτ,ss, C max,ss and renal clearance (CLR,0–24,ss) of digoxin were all within the bioequivalence range 80–125%, which is important as digoxin has a narrow therapeutic range. There was a low incidence of adverse events, which were randomly distributed between treatment groups. In conclusion, linagliptin did not alter the pharmacokinetics of digoxin in this study, indicating that linagliptin does not inhibit P-glycoprotein or other transporters relevant for digoxin pharmacokinetics. These results suggest that linagliptin and digoxin can be co-administered without dose adjustment. Administration of digoxin alone and with linagliptin was well tolerated.


Similar content being viewed by others
References
Aspen Europe GmbH (2010) Lanoxin 125 Tablets, Summary of Product Characteristics (last updated 8 March 2010). http://www.medicines.org.uk/EMC/medicine/2174/SPC/Lanoxin+125+Tablets/. Accessed 17 Nov 2010
Balimane PV, Chong S (2005) A combined cell based approach to identify P-glycoprotein substrates and inhibitors in a single assay. Int J Pharmacol 301:80–88
Barnett AH, Harper R, Toorawa R, Patel S, Woerle H-J (2010) Linagliptin monotherapy improves glycaemic control in type 2 diabetes patients for whom metformin therapy is inappropriate. Diabetologia 53(Suppl 1):S327 (Poster 823, presented at the European Association for the Study of Diabetes 46th Annual Meeting, Stockholm, Sweden. 20–24 September 2010)
Blech S, Ludwig-Schwellinger E, Gräfe-Mody EU, Withopf B, Wagner K (2010) The metabolism and disposition of the oral dipeptidyl peptidase-4 inhibitor, linagliptin, in humans. Drug Metab Dispos 38:667–678
Deacon CF, Holst JJ (2010) Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Investig Drugs 19:133–140
Del Prato S, Barnett AH, Huisman H, Neubacher D, Woerle H-J, Dugi KA (2011) Effect of linagliptin monotherapy on glycaemic control and markers of β-cell function in patients with inadequately controlled type 2 diabetes: a randomised controlled trial. Diabetes Obes Metab 13:258–267
Eckhardt M, Langkopf E, Mark M, Tadayyon M, Thomas L, Nar H, Pfrengle W, Guth B, Lotz R, Sieger P, Fuchs H, Himmelsbach F (2007) 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3, 7-dihydropurine-2, 6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J Med Chem 50:6450–6453
FDA Draft Guidance on Drug Interactions (2006) US FDA Center for Biologics Evaluation and Research (CBER), September 2006. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093606.htm. Accessed 17 Nov 2010
Forst T, Uhlig-Laske B, Ring A, Graefe-Mody U, Friedrich C, Herbach K, Woerle H-J, Dugi KA (2010) Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabet Med 12:1409–1419
Fromm MF (2000) P-glycoprotein: a defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int J Clin Pharmacol Ther 38:69–74
Gomis R, Espadero R-M, Jones R, Woerle H-J, Dugi KA (2010) Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes. Poster 551-P, presented at the 70th scientific sessions of the American diabetes association, Orlando, Florida, June 25–29, 2010. Abstract available at: http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79499. Accessed 17 Nov 2010
Graefe-Mody EU, Padula S, Ring A, Withopf B, Dugi KA (2009) Evaluation of the potential for steady-state pharmacokinetic and pharmacodynamic interactions between the DPP-4 inhibitor linagliptin and metformin in healthy subjects. Curr Med Res Opin 25:1963–1972
Graefe-Mody U, Huettner S, Stähle H, Ring A, Dugi KA (2010a) Effect of linagliptin (BI 1356) on the steady-state pharmacokinetics of simvastatin. Int J Clin Pharmacol Ther 48:367–374
Graefe-Mody U, Friedrich C, Port A, Ring A, Heise T, Halabi A, Woerle H-J (2010b) Linagliptin, a novel DPP-4 inhibitor: no need for dose adjustment in type 2 diabetes patients with renal impairment. Diabetologia, 53(Suppl 1): S326. Poster 822, presented at the European association for the study of diabetes 46th annual meeting, Stockholm, Sweden, 20–24 Sept 2010.
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234
Hayward R (1987) Digitalis: the present position. In: Hamer J (ed) Drugs for heart disease, 2nd edn. Chapman and Hall, London, pp 145–193
He YL, Sabo R, Sunkara G, Bizot MN, Rivierie GJ, Leon S, Ligueros-Saylan M, Dole WP, Howard D (2007) Evaluation of pharmacokinetic interactions between vildagliptin and digoxin in healthy volunteers. J Clin Pharmacol 47:998–1004
Heise T, Graefe-Mody EU, Hüttner S, Ring A, Trommeshauser D, Dugi KA (2009) Pharmacokinetics, pharmacodynamics and tolerability of multiple oral doses of linagliptin, a dipeptidyl peptidase-4 inhibitor in male type 2 diabetes patients. Diabetes Obes Metab 11:786–794
Horn JR, Hansten PD (2004) Drug interactions with digoxin: the role of P-glycoprotein. Pharmacy Times. http://www.hanstenandhorn.com/hh-article10-04.pdf. Accessed 17 Nov 2010
Hüttner S, Graefe-Mody EU, Withopf B, Ring A, Dugi KA (2008) Safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of BI 1356, an inhibitor of dipeptidyl peptidase 4, in healthy male volunteers. J Clin Pharmacol 48:1171–1178
Karim A, Fleck P, Harris S, Weiss M, Zhang W, Mekki Q (2008) Lack of pharmacokinetic interaction between multiple doses of the dipeptidyl peptidase-4 inhibitor alogliptin and digoxin in healthy subjects. Clin Pharmacol Ther 83:S12–13 (Presented at the American society for clinical pharmacology and therapeutics, Orlando, Florida, April 2–5, 2008)
Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, Yoshiharu H, Sarashina A, Woerle H-J, Dugi KA (2010a) Linagliptin monotherapy improves glycemic control in Japanese patients with T2DM over 12 weeks. Poster 696-P, presented at the 70th scientific sessions of the American diabetes association, Orlando, Florida, June 25–29, 2010. Abstract available at: http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79641. Accessed 17 Nov 2010
Kawamori R, Inagaki N, Araki E, Watada H, Hayashi N, Horie Y, Sarashina A, Woerle H-J, Dugi KA (2010b) Linagliptin provides superior glycemic control compared to voglibose as monotherapy in Japanese patients with type 2 diabetes. Poster 632-P, presented at the 70th scientific sessions of the American diabetes association, Orlando, Florida, June 25–29, 2010. Abstract available at: http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79579. Accessed 17 Nov 2010
Kim RB (2003) Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest 33(Suppl 2):1–5
Lewin AJ, Arvay L, Liu D, Patel S, Woerle H-J (2010) Safety and efficacy of linagliptin as add-on therapy to a sulphonylurea in inadequately controlled type 2 diabetes. Diabetologia 53(Suppl 1):S326. Poster 821, presented at the European association for the study of diabetes 46th annual meeting, Stockholm, Sweden, 20–24 Sept 2010
Owens DR, Swallow R, Jones P, Dugi KA, Woerle H-J (2010) Linagliptin improves glycemic control in type 2 diabetes patients inadequately controlled by metformin and sulfonylurea without weight gain or hypoglycemia. Poster 548-P, presented at the 70th scientific sessions of the American diabetes association, Orlando, Florida, June 25–29, 2010. Abstract available at: http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79496. Accessed 17 Nov 2010
Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM (2003) Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA 289:871–878
Retlich S, Withopf B, Greischel A, Staab A, Jaehde U, Fuchs H (2009) Binding to dipeptidyl peptidase-4 determines the disposition of linagliptin (BI 1356)—investigations in DPP-4 deficient and wildtype rats. Biopharm Drug Dispos 30:422–436
Schwartz JI, Agrawal NG, Wehling M, Musser BJ, Gumbs CP, Miechiels N, De Smet M, Wagner JA (2008) Evaluation of the pharmacokinetics of digoxin in healthy subjects receiving etoricoxib. Br J Clin Pharmacol 66:811–817
Sechaud R, Robeva A, Belleli R, Balez S (2008) Absence of an effect of a single-dose deferasirox on the steady-state pharmacokinetics of digoxin. Int J Clin Pharmacol Ther 46:519–526
Tahrani AA, Piya MK, Barnett AH (2009) Saxagliptin: a new DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Adv Ther 26:249–262
Taskinen M-R, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, Woerle H-J (2011) Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled study. Diabetes Obes Metab 13:65–74
Acknowledgments
The authors would like to thank the volunteers and staff who participated in this study. This study was sponsored and funded by Boehringer Ingelheim. All of the authors are employees of Boehringer Ingelheim. Bioanalytical support for this manuscript was provided by Dr. Frank Runge. Medical writing and editorial support for the manuscript was provided by Alice Walmesley of PHASE II International Ltd with financial support from Boehringer Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedrich, C., Ring, A., Brand, T. et al. Evaluation of the pharmacokinetic interaction after multiple oral doses of linagliptin and digoxin in healthy volunteers. Eur J Drug Metab Pharmacokinet 36, 17–24 (2011). https://doi.org/10.1007/s13318-011-0028-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-011-0028-y