Abstract
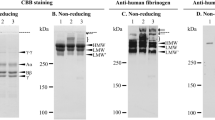
Fibrinogen is a 340-kDa plasma protein that is composed of two identical molecular halves, each consisting of three non-identical subunit polypeptides designated as Aa, Bβ- and λ-chains held together by multiple disulfide bonds. Fibrinogen has a trinodular structure, i.e., one central E domain comprizing the amino-terminal regions of paired individual three polypeptides, and two identical outer D domains. These three nodules are linked by two coiled-coil regions [1,2]. After activation with thrombin, a tripeptide segment consisting of Gly-Pro-Arg is exposed at the amino-terminus of each α-chain residing at the center of the E domain and combines with its complementary binding site, called the ‘a’ site, residing in the carboxyl-terminal region of the γ-chain in the outer D domain of another molecule. By crystallographic analysis [3], the α-amino group of αGly-1 is shown to be juxtaposed between the carboxyl group of γAsp-364 and the carboxyamide of Gln-329 in the ‘a’ site. Half molecule-staggered, double-stranded fibrin protofibrils are thus formed [4,5]. Upon abutment of two adjacent D domains on the same strand, D-D self association takes place involving Arg-275, Tyr-280 and Ser-300 of the γ-chain on the surface of the abutting two D domains [3]. Thereafter, carboxyl-terminal regions of the fibrin α-chains are thought to be untethered and interact with those of other protofibrils leading to the formation of thick fibrin bundles and interwoven networks after appropriate branching [6–9]. Although many enigmas still remain regarding the mechanisms of these molecular interactions, fibrin assembly proceeds in a highly ordered fashion. In my talk, I would like to discuss these molecular interactions of fibrinogen and fibrin based on the up-date data provided by analyses of normal as well as hereditary dysfibrinogens, particularly in the latter by introducing representative molecules at each step of fibrin clot formation.
Similar content being viewed by others
References
Doolittle RE, H. Bouma Iii, BA Cottrell, et al. The covalent structure of human fibirinogen. In the chemistry and physiology of the human plasma proteins. D.H. Bing, Ed.: Pergamon Press, New York. 1979. p77–95.
Doolittle RE. Fibirinogen and fibrin. In Haemostasis and Thrombosis, 2nd edit. A.L. Bloom & D.P. Thomas, Eds.: Churchil Livingstone, Edinburgh. 1981. p163–191.
Spraggon G, Everse S, Doolittle RE Crystal structures of fragment D from human fibrinogen and its crosslinked counterpart from fibrin.Nature. 1997;389:455–462.
Cote HCE, Pratt KP Davie EW, Chung DW. The polymerization pocket ‘a’ within the carboxyl-terminal region of the γ chain of human fibrinogen is adjacent to but independent from the calcium-binding site.J Biol Chem. 1977;272:23792–23798.
Everse SJ, Spraggon G, Doolittle FE. A three dimensional consideration of varian human fibrinogens.Thromb Haemost. 1998;80:1–9.
Weisel JW. Lateral aggregation and the role of the two pairs of fibrinopeptides.Biophys J. 1986;50:1079–1093.
Weisel JW, Veklich Y, Gorkun O. The sequence of cleavage of fibrinopeptides from fibrinogen is important for protofibril formation and enhancemenet of lateral aggregation in fibrin clots.J Mol Biol. 1993;232:285–297.
Gorkun OV, Veklich YI, Medved LV, et al. Role of the αC domains of fibrin in clot formation.Biochemistry. 1994;33: 6986–6997.
Veklich LV, Gorkun OV, Medved LV, et al. Carboxyl-terminal portions of the α chains of fibrinogen and fibrin. Localization by electron microscopy and the effects of isolated α fragments on polymerization.J Biol Chem. 1993; 268:13577–13585.
Matsuda M, Sugo T, Yoshida N, et al. Structure and function of fibrinogen: insights fromdysfibrinogens.Thromb Haemost. 1999;82:291–297.
Matsuda M. Structure and function of fibrinogen inferred from hereditary dysfibrinogens.Fibrinolysis & Proteolysis. 2000;14:187–197.
Matsuda M, Sugo T. Hereditary disorders of fibrinogen.Anal N.Y. Acad Sci. 2001;936:65–88.
Mosesson MW, Diorio JP, Siebenlist KR, et al. Evidence for a second type of fibrin branch point in fibrin polymer networks, the trimolecular branch junction.Blood. 1993;82:1517–1521.
Baradet TC, Haselgrove JC, Weisel JW. Three-dimensional reconstruction of fibrin clot networks from stereoscopic intermediate voltage electron microscopic images and analysis of branching.Biophys J. 1995;68:1551–1560.
Yee VC, Pratt KP, Cote HCF, et al. Crystal structure of a 30 kDa C-terminal fragment from the γchain of human fibrinogen.Structure. 1997;5:125–138.
Pratt KP, Cote HCF, Chung DW, et al. The fibrin polymerization pocket; three-dimension structure of a 30 kDA C-terminalγchain fragment complexed with the peptide Gly-Pro-Arg-Pro.Proc Atl Acad Sci. U.S.A. 1997;94:7176–7181.
Southan C. The elucidation of molecular defects in congenital dysfibrinogenemia. In: Fibrinogen, Fibrin Stabilization, and Fibrinolysis. J.L. Francis, Ed.: 1998. p199–127.
Niwa K, Yaginuma A, Nakanishi M, et al. Fbirinogen Mitaka II: a hereditary dysfibrinogen with defective thrombin binding caused by an Aα Glu-11 to Gly substitution.Blood. 1993;82:3658–2663.
Martin PD, Robertson W, Turk D, et al. The structure of residues 7–16 of the Aα-chain of human fibrinogen bound to bovine thrombin at 2.3-Å resolution.J Biol Chem. 1992;267:7911–7920.
Stubb MT, Aschkinant H, Mayer I, et al. The interaction of thrombin with fibirinogen. A structural basis for its specificity.Eur J Biochem. 1992;206:187–195.
Zheng Z, Ashton RW, Ni F, Scheraga HA. Thrombin hydrolysis of an N-terminal peptide from fibirinogenn Lille: kinetic and NMR studies.Biochemistry. 1992;31:4426–4431.
Higgins DL, Shafer JA. Fibirinogen Petoskey, a dysfibirinogenemia characterized by replacement of Arg-Aα16 by a histidy residue. Evidence for thrombincatalyzed hydrolysis at a histidyl residue.J Biol Chem. 1981;256:12013–12017.
Henschen A, Kehl M, Southan S. Genetically abnormal fibinogens-strategies for structure elucidation, including fibirinopeptide analysis. In: Variants of Human Fibrinogen E.A. Beck & M. Furlan. Eds.: Hans Huber Bverlag, Bern. 1984. p273–320.
Matsuda M. Molecular abnormalities of fibrinogen-the present status of structure elucidation. In: Fibirinogen 4. Current Basis and Clinical Aspects. M. Matsuda, S. Iwanaga, A. Takada & A. Henschen, Eds. Excerpta Medica, Amsterdam. 1990. p139–152.
Galanakis D. Ingerited dysfibrinogenemia: emerging abnormal structure associations with pathologic and nonpathologic dysfunctions.Semin Throm Hemostas. 1993;19:386–395.
Kudryk BJ, Collen D, Woods KR, Blombck. evidence for localization of polymerization sites infibrinogen.J Biol Chem. 1974;249:3322–3325.
Olexa SA, Budzynski AZ. Evidence for four different polymerization sites involved in human fibrin formation.Proc Natl Acad Sci USA. 1980;77:1374–1378.
Mosesson MW, Siebenlist KR, Dioliio JP, et al. The role of fibrinogen D domain intermolecular association sites in the plymerization of fibrin and fibrinogen Tokyo II (γ275 Arg→Cys).J Clin Invest. 1995;96:1053–1058.
Laudano AP, Doolittle RF. Synthetic peptide derivatives that bind to fibirinogen and prevent the plymerization of fibrin monomers.Proc Natl Acad Sci. U.S.A. 1978;75:3085–3089.
Ludano AP, Dollittle RF. Studies on synthetic peptides that bind to fibirinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences.Biochemistry. 1980;19:1013–1019.
Wada Y, Niwa K, Maekawa H, et al. A new type of congenital dysfibrinogen, fibrinogen Bremen, with an Aα Gly-17 to Val substitution associated with hemorrhagic diathesis and delayed wound healing.Thromb Haemost. 1993;70:397–403.
Yoshida N, Okuma MO, Hirata H, et al. Fibrinogenn Kyoto II, a new congenitally abnormal molecule, characterized by the replacement of Aα proline-18 by leucine.Blood. 1991; 78:149–153.
Uotani C, Miyata T, Kumabashiri I, et al. Fibrinogen Kanazawa: a congenital dysfibirinogenemia with delayed polymerization having a replacement of proline-18 by leucine in the A α-chain.Blood Coag Fibrinol. 1991;2:413–417.
Blonb · Ck, M, Blonb · Ck, EF Mammen, Prasad AS. Fibiriogen Detroit-A molecular defect in the N-terminal disulphide knot of human fibrinogen?Nature. 1968;218:134–137.
Hessel B, Stenbjerg S, Dyr J, et al. Fbirinogen Aarhus-a new case of dysfibrinogenemia.Thromb Res. 1986;42:21–37.
Dempfle CEH, Henschen A. Fibrinogen Mannheim I-identification of an Aα C19 Arg→Gly substitution in dysfibrinogenemia associated with bleeding tendency. In: Fibrinogen 4. Current Basic and Clinical Aspects. Matsuda M, Iwanaga S, Takada A, Henschen A, Eds. Elevier Science Publ. Amsterdam. 1990, p159–166.
Yamaguchi S, Sugo T, Hashimoto Y, et al. Fibrinogen Kumamoto with an Aα Arg-19 to Gly substituion has reduced affinity for thrombin: possible relevance to thrombosis. Jpn.J Throm Haemost. 1997;8:382–392.
Miyata T, Furukawa K, Iwanaga S, et al. Fibrinogen Nagoya, a replacement of glutamine-329 by arginine in the γ-chain that impairs the polymerization of fibirin monomer.J Biochem. 1989;105:10–14.
Reber P, Furlan M, Rupp C, et al. Characterizatioon of fibirinogen Milano I: amino acid exchangeγ330 Asp→Val impairs fibrin polymerization.Blood. 1986;67:1751–1756.
Terukina S, Yamazumi K, Okamoto K, et al. Fibirinogen Kyoto III: a congenital daysfibrinogen with a γ aspartic acid-330 to tyrosine substitution manifesting impaired fibrin monomer polymerization.Blood. 1989;74:2681–2687.
Okamura N, Furihata K, Terasawa F, et al. Fibirinogen Matsumoto I: aγ 364 Asp→His. (GAT→CAT) substitution associated with defective fibrin polymerization.Thromb Haemost. 1996;75:887–891.
Bentolia S, Samama MM, Conard J, et al. Association of dysfibrinogenemia and thrombosis. Apropos of a family (fibirinogen Melun) and review of the literature (in French).Annalen Med Interne. 1995;146:575–580.
Ct HCF, Lord ST, Pratt KP. γ-chain dysfibrinogenemias: molecular structure-function relationships of naturally occurring mutations in the γ chain of human fibrinogen.Blood. 1998; 92:2195–2212.
Yoshida N, Hirata H, Morigami Y, et al. Characterization of an abnormal fibirinogen Osaka V with the replacement of γ-arginine 375 by glycine.J Biol Chem. 1992;267:2753–2759.
Steinmann C, Rebver P, Jungo M, et al. Fibirinogen Bern I: substitutionγ337 Asn→Lys is responsible for defective fibrin monomer polymerization.Blood. 1993;82:2104–2108.
Steinmann C, Bgli C, Jungo M, et al. A new substitution, γ 358 Ser→Cys, in fibrinogen Milano VII causes defective fibrin polymerization.Blood. 1994;84:1874–1880.
Matsuda M, Nakamikawa C, Baba M, Morimoto K. Fibrinogen Tokyo II: an abnormal fibirinogen with an impaired polymerization site on the aligned DD domain of fibrin molecules.J Clin Invest. 1983;72:1034–1041.
Terukina S, Matsuda M, Hirata H, et al. Substitution of γ Arg-275 by Cys in an abnormal fibrinogen. Fibrinogen Osaka II. Evidence for a unique solitary systine structure at themutation site.J Biol Chem. 1988;263:13579–13587.
Reber P, Furlan M, Henschen A, et al. Three abnormal fibirnogen variants with the same amino acid substitution (γ 275 Arg→His): fibrinogens Bergamo II, Essen and Perugia.Thromb Haemost. 1986;56:401–406.
Yamazumi K, Terukina S, Onohara S, Matsuda M. Normal plasmic cleavge of the γchain variant of fibrinogen Saga with an Arg-275 to His substitution.Thromb Haemost. 1988; 60:476–480.
Mimuro J, Kawata Y, Niwa K, et al. A new type of Ser substitution for γArg-275 in fibrinogen Kamogawa I characterized by impaired fibrin assembly.Thromb Haemost. 1999; 81:940–944.
Fellowes AP, Brennan SO, Ridgway HJ, et al. Electrospray ionization mass spectrometry identification of fibrinogen Banks Peninsula (γ280 Tyr→Cys): a new variant with defective polymerization.Brit Haemost. 1998;101:24–31.
NIWA K, TAKEBE M, SUGO T, et al. A γGly-268 to Glu substitution is responsible for impaired fibrin assembly in a homozygous dysfibrinogen Kurashiki I.Blood. 1996;87:4686–4694.
Yoshida N, Terukina S, Okuma M, et al. Characterization of an apparently lower molecular weight γ-chain variant in fibrinogen Kyoto I. The replacement of γ Asn-308 by Lys which caused an accelerated cleavage of fragment DI by plasmin and the generation of a new plasmin cleavage site.J Biol Chem. 1998;263:13949–13856.
Bantia S, Bell WR, Dang CV. Polymerization defect of fibirinogen Baltimore III due to a gamma Asn-308→Ile mutation.Blood. 1990;75:1659–1663.
Yamazumi K, Shimura K, Terukina S, et al. Aγ aspargine-308 identified in a congenital dysfibrinogenemia associated with posttraumatic bleeding, fibrinogen Asahi.J Clin Invest. 1989;83:1590–1597.
Sugo T, Nakamikawa C, Yoshida N, et al. End-linked homodimers in fibrinogen Osaka VI with a Bβ-chain extension lead to fragile clot structure.Blood. 2000;96:3779–3785.
Townsend RR, Hilliker E, Li YT, et al. Carbohydrate structure of human fibirinogen.J Biol Chem. 1982;257:9704–9710.
Koopman J, Haverkate F, Grimbergen J, et al. Fibrinogen Marburg: a homozygous case of dysfibrinogenemia, lacking amino acids Aα461-610(Lys 461AAA→Stop TAA).Blood. 1992;80:1972–1979.
Sugo T, Nakamikawa C, Takebe M, et al. Factor XIIIa-cross-linking of the Marburg Fibrin: Formation of αm v n-heteromultimers and the α-chain-linked albumin v complex, and disturbed protofibril assembly resulting in acquisition of plasmin-resistance relevant to thrombophilia.Blood 1998;91: 3282–3288.
Koopman J, Haverkate F, Grimbergen J, et al. Molecular basis for fibrinogen Dusart (Aα554 Arg→Cys) and its association with abnormal fibrin polymnerization and thrombophilia.J Clin Invest. 1993;91:1637–1643.
Wada Y, Lord ST. A correlation between thrombotic disease and a specific fibirinogen abnormality (Aα 554 Arg→Cys)in two unrelated kindred, Dusart and Chapel Hill III.Blood. 1994;84:3709–3714.
Maekawa H, Yamazumi K, Muramatsu S, et al. An Aα Ser-434 to N-glycosylated Asn substitution in a dysfibrinogen, fibrinogen Caracas II, characterized by impaired fibrin gel formation.J Biol Chem. 1991;266:11575–11581.
Collet JP, Woodhead JL, Soria J, et al. Fibrinogen Dusart; electron microscopy of molecules, fibers and clots, and viscoelastic properties of clots.Biophys J. 1996;70:500–510.
Mosesson MW, Sievenlist KR, Hainfeld JF, et al. The relationship between the fibrinogen D domain self-association/cross-linking site (γXL) and the fibirinogen Dusart abnormality (AαR554C-albumin). Clues to thrombophilia in the Dusart syndrome,J Clin Invest. 1996;97:2342–2350.
Woodhead JL, Nagaswami C, Matsuda M, et al. The ultrastructure of fibirinogen Caracas II molecules, fibers and clots.J Biol Chem. 1996;271:4946–4953.
Maekawa H, Yamazumi K, Muramatsu S, et al. Fibrinogen Lima: a homozygous dysfibirinogen with an Aα-arginine-141 to serine substitution associated with extra N-glycosylation at Aα-asparagine-139.J Clin Invest. 1992;90:67–76.
Ridgway HJ, Brennan SO, Loreth RM, George PM. Fbirinogen Kaiserslautern (γ380 Lys to Asn): A new glycosylated fibrinogen variant with delayed polymrization.Br J Haematol. 1997;99:562–569.
Marguerie G, Chagneil G, Suscilion M. The binding of calcium to bovine fibrinogen.Biochim Biophys Acta 1977; 490: 94–103.
Koopman J, Haverkate F, BriËte E, Lord ST. A congenitally abnormal fibirinogen (Vissingen) with a 6-base delation in the γchain gene, causing defective calcium binding and impaired fibrin polymerization.J Biol Chem 1991;266: 13456–13461.
Suenson E, Bjerrum P, Holm A, et al. The role of fragment X plymers in the fibrin enhancement of tissue plasminogen activator-catalyzed plasmin formation.J Biol Chem. 1990;265:2228–22237.
Lijnen HR, Soria J, Soria C, et al. Dysfibrinogenemia (Fibrinogen Dusart) associated with impaired fibrin-enhanced plasminogen activation.Thromb Haemost. 1984;51:108–109.
Carrell N, Gabriel DA, Blatt PM, et al. Heteditary dysfibrinogenemia in a patient with thrombotic disease.Blood. 1983;62:439–447.
Author information
Authors and Affiliations
About this article
Cite this article
Matsuda, M., Sugo, T. Structure and function of human fibrinogen inferred from dysfibrinogens. Int J Hematol 76 (Suppl 1), 352–360 (2002). https://doi.org/10.1007/BF03165284
Issue Date:
DOI: https://doi.org/10.1007/BF03165284