Summary
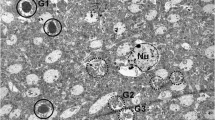
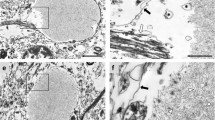
Abnormal granular structures, which stained positively with periodic acid-Schiff (PAS-positive granular structures; PGS), were observed in the brain of senescence accelerated mouse (SAM). They were small, round to ovoid, homogenous structures measuring up to 5 μm in diameter and usually grouped in clusters. PGS were localized in the hippocampus, piriform cortices, olfactory tubercle, nucleus of the trapezoid body, and cerebellar cortices. Quantitative analysis revealed that PGS remarkably increased in the hippocampus of SAM-P/8, a substrain of SAM, with advancing age, although a few PGS also appeared in the aged control mice, SAM-R/1 and DDD. Their histochemical nature, morphological features and distribution pattern were different from those of corpora amylacea and other similar bodies. A close anatomical relationship between PGS and glial fibrillary acidic protein-positive astrocytes was inferred from immunohistochemical studies. PGS is considered to be one of the morphological manifestations of senescence in mice brains, and are found to occur more numerously in the brains of learning or memory deficit mice, SAM-P/8.
Similar content being viewed by others
References
Blumer D (1959) Dimedone as an aldehyde blocking reagent to facilitate the histochemical demonstration of glycogen. Stain Technol 34:95–98
Fraser H (1969) Eosinophilic bodies in some neurons in the thalamus of ageing mice. J Pathol 98:201–204
Higuchi K, Matsumura A, Hunma A, Takeshita S, Hashimoto K, Hosokawa M, Yasuhira K, Takeda T (1983) Systemic senile amyloid in senescence-accelerated mice. Lab Invest 48:231–240
Hosokawa M, Kasai R, Higuchi K, Takeshita S, Shimizu K, Hamamoto H, Honma A, Irino M, Toda K, Matsumura A, Matsushita M, Takeda T (1984a) Grading score system: a method for evaluation of the degree of senescence in senescence accelerated mouse (SAM). Mech Ageing Dev 26:91–102
Hosokawa M, Takeshita S, Higuchi K, Shimizu K, Irino M, Toda K, Honma A, Matsumura A, Yasuhira K, Takeda T (1984b) Cataract and other ophthalmic lesions in senescence accelerated mouse (SAM). Morphology and incidence of senescence associated ophthalmic changes in mice. Exp Eye Res 38:105–114
Kimura H, McGeer PL, Peng JH, McGeer EG (1981) The central cholinergic system studied by choine acetyltransferase immunohistochemistry in the cat. J Comp Neurol 200:151–201
Matsushita M, Tsuboyama T, Kasai R, Okumura H, Yamamuro T, Higuchi Ke, Higuchi Ka, Yonezu T, Utani A, Umezawa M, Takeda T (1986) Age-related changes in bone mass in the senescence accelerated mouse (SAM): SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol (in print)
Miyamoto M, Kiyota Y, Yamazaki N, Nagaoka A, Matsuo T, Nagawa Y, Takeda T (1986) Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol Behav (in print)
Nakamura Y, Okamoto M (1973) An electron microscopic study of aging alterations in the mouse brain. Adv Neurol Sci 17:647–658
Nandy K (1966) Effect of centrophenoxine on the lipofuscin pigments in the neurons of senile guinea pigs. Nature 210:313–314
Okamoto K, Ueda M, Kusumoto Y, Hashimoto M (1948) The histochemical test-method of cerebrosic. Jpn J Const Med 14:27–29
Onteniente B, Kimura H, Maeda T (1983) Comparative study of the glial fibrillary acidic protein in vertebrates by PAP immunohistochemistry. J Comp Neurol 215:427–436
Puchtler H, Sweat F, Levine M (1962) On the binding of Congo red by amyloid. J Histochem Cytochem 10:355–364
Ramsay HJ (1965) Ultrastructure of corpora amylacea. J Neuropathol Exp Neurol 24:25–39
Sternberger LA (1979) Immunohistochemistry, 2nd edn. John Wiley and Sons, New York
Sakai M, Austin J, Witmer F, Trueb L (1969) Studies of corpora amylacea. Arch Neurol 21:526–544
Suzuki Y, Akiyama K, Suu S (1978) Lafora-like inclusion bodies in the CNS of aged dogs. Acta Neuropathol (Berl) 44:217–222
Suzuki Y, Kamiya S, Ohta K, Ohta K, Suu S (1979) Lafora-like bodies in a cat. Acta Neuropathol (Berl) 48:55–58
Takeshita S, Hosokawa M, Irino M, Higuchi K, Shimizu K, Yasuhira K, Takeda T (1982) Spontaneous age-associated amyloidosis in senescence-accelerated mouse (SAM). Mech Ageing Dev 20:13–23
Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsuhita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamura T (1981) A new murine model of accelerated senescence. Mech Ageing Dev 17:183–194
Author information
Authors and Affiliations
Additional information
Supported by grants from the Japanese Ministry of Education, Culture and Science and the Ministry of Health and Welfare
Rights and permissions
About this article
Cite this article
Akiyama, H., Kameyama, M., Akiguchi, I. et al. Periodic acid-Schiff (PAS)-positive, granular structures increase in the brain of senescence accelerated mouse (SAM). Acta Neuropathol 72, 124–129 (1986). https://doi.org/10.1007/BF00685973
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685973