Abstract
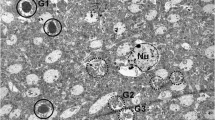
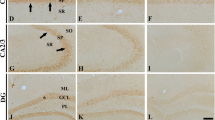
We examined the histochemical localization of monoamine oxidase in the hippocampus of young and old senescence-accelerated mouse (SAM). We found a monoamine oxidase-B-positive granular structure (MGS) in the hippocampus of old SAMP8, an accelerated senescenceprone line of SAM. The MGS was a round-shaped granular structure of 0.5 to 5 μm diameter and usually formed a cluster, the largest diameter of which ranged from 50 to 150 μm. No MGS were found in the hippocampus of young SAMP8 or of young SAMR1, an accelerated senescence resistant line of SAM, and only few, if any, were seen in old SAMR1. A monoamine oxidase-positive astrocyte was usually observed in the central area of each cluster of MGS. Furthermore, the MGS was in close anatomical relationship with monoamine oxidase-positive astrocytic processes. The enzyme inhibition experiments showed that monoamine oxidase activities localized in the MGS and astrocytes were both predominantly of type B. These findings suggest MGS occurs at least partly in monoamine oxidase-B-positive astrocytes. Furthermore, the MGS was similar to a periodic acid-Schiff-positive granular structure, a polyglucosan body previously documented in the brains of old SAMP8 and some other aged mice strains including C57BL/6 and nude mice, in terms of their size, morphological appearances and topographical distribution in the hippocampus. Thus, the present results suggest that monoamine oxidase type B is a proteinaceous component of the periodic acid-Schiff-positive granular structure in aged mice brains, and might provide some clues for clarifying the mechanisms of age-related occurrence of periodic acid-Schiff-positive granular structures in mice brains.
Similar content being viewed by others
References
Adolfsson R, Gottfries C-G, Oreland L, Wiberg A, Winblad B (1980) Increased activity of brain and platelet monoamine oxidase in dementia of Alzheimer type. Life Sci 27: 1029–1034
Akiyama H, Kameyama M, Akiguchi I, Sugiyama H, Kawamata T, Fukuyama H, Kimura H, Matsushita M, Takeda T (1986) Periodic acid-Schiff (PAS)-positive granular structures increase in the brain of senescence acceleaated mouse (SAM). Acta Neuropathol (Berl) 72: 124–129
Arai R, Kumura H, Maeda T (1986) Topographic atlas of monoamine oxidase-containing neurons in the rat brain studied by an improved histochemical method. Neuroscience 19: 905–925
Arai Y, Kinemuchi H (1988) Differences between MAO concentrations in striatum and forebrain of aged and young rats. J Neural Transm 72: 99–105
Arai Y, Stenström A, Oreland L (1985) The effect of age on intra- and extraneuronal monoamine oxidase −A and −B activities in the rat brain. Biogenic Amines 2: 65–71
Bach AWJ, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan S-W, Seeburg PH, Shih JC (1988) cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci USA 85: 4934–4938
Chen WH, Hosokawa M, Tsuboyama T, Ono T, Iizuka T, Takeda T (1989) Age-related changes in the temporomandibular joint of the senescence accelerated mouse. Am J Pathol 135: 379–385
Fowler CJ (1982) Selective inhibitors of MAO types A and B and their clinical usefulness. Drug Future 7: 501–517
Fowler CJ, Tipton KF (1984) On the substrate specificities of the two forms of monoamine oxidase. J Pharm Pharmacol 36: 111–115
Fowler CJ, Oreland L, Marcusson J, Winblad B (1980) Titration of human brain monoamine oxidase-A and-B by clorgyline and L-deprenyl. Naunyn-Schmiedebergs Arch Pharmacol 311: 263–272
Fowler CJ, Wiberg A, Oreland L, Marcusson J, Winblad B (1980) The effect of age on the activity and molecular properties of human monoamine oxidase. J Neural Transm 49: 1–20
Garrick NA, Murphy DL (1980) Species differences in the deamination of dopamine and other substrates for monoamine oxidase in brain. Psychopharmacology 72: 27–33
Glover V, Sandler N, Owen F, Riley GT (1977) Dopamine is a monoamine oxdiase B substrate in man. Nature 265: 80–81
Gottfries CG, Oreland L, Wiberg A, Winblad B (1975) Lowered monoamine oxidase activity in brains from alcoholic suicides. J Neurochem 25: 667–673
Higuchi K, Yonezu T, Kogichi K, Matsumuara A, Takeshita S, Higuchi K, Kohno A, Matsushita M, Hosokawa M, Takeda T (1986) Purification and charaterization of a senile amyloid-related antigenic substance (apoSASSAM) from mouse serum. J Biol Chem 261: 12834–12840
Hosokawa M, Takeshita S, Higuchi K, Shimizu K, Irino M, Toda K, Honma A, Matsumura A, Yasuhira K, Takeda T (1984) Cataract and other ophthalmic lesions in senescence accelerated mouse (SAM). Morphology and incidence of senescence associated ophthalmic changes in mice. Exp Eye Res 38: 105–114
Johnston JP (1968) Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol 17: 1285–1297
Jossan SS, Gillberg GG, Gottfries CG, Karlsson I, Oreland L (1989) MPTP toxicity in relation to age, dopamine uptake and MAO-B activity in two rodents species. Pharmacol Toxicol 64: 314–318
Jucker M, Ingram DK (1994) Age-related fibrillar material in mouse brain: assessing its potential as a biomarker of aging and as a model of human neurodegenerative disease. Ann NY Acad Sci 719: 238–247
Jucker M, Walker LC, Martin LJ, Kitt CA, Kleinman HK, Ingram DK, Price DL (1992) Age-associated inclusions in normal and transgenic mouse brain. Science 255: 1443–1445
Jucker M, Walker LC, Schwarb P, Hengmihle J, Kuo H, Snow AD, Bamert F, Ingram DK (1994) Age-related deposition of glia-associated fibrillar material in brains of C57 BL/6 mice. Neuroscience 60: 875–889
Kawamata T, Nakamura S, Akiguchi I, Kimura J, Kameyama M, Kimura H, Takeda T (1990) Effect of aging on NADPH-diaphorase neurons in laterodorsal tegmental nucleus and striatum of senescence accelerated mouse (SAM). In: Nagatsu T (ed) Basic, clinical, and therapeutic aspects of Alzheimer's and Parkinson's disease, vol 1. Plenum Press, New York, pp 705–709
Kishimoto S, Kimura H, Maeda T (1983) Histochemical demonstration for monoamine oxidase (MAO) by a new coupled peroxidation method. Cell Mol Biol 29: 61–69
Kitahama K, Denney RM, Maeda T, Jouvet M (1991) Distribution of type B monamine oxidase immunoreactivity in the cat brain with reference to enzyme histochemistry. Neuroscience 44: 185–204
Knoll J, Magyar K (1972) Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol 5: 393–408
Konradi C, Svoma E, Jellinger K, Riederer P, Denney R, Thibault J (1988) Topographic immunocytochemical mapping of monoamine oxidase-A, monoamine oxidase-B and tyrosine hydroxylase in human post mortem brainstem. Neuroscience 26: 791–802
Kornhuber J, Konradi C, Mack-Burkhardt F, Riederer P, Heinsen H, Beckmann H (1989) Ontogenesis of monoamine oxidase-A and-B in the human brain frontal cortex. Brain Res 499: 81–86
Lamar CH, Hinsman EJ, Herikson CK (1976) Alterations in the hippocampus of aged mice. Acta Neuropathol (Berl) 36: 387–391
Leung TKC, Lai JCK, Lim L (1981) The regional distribution of monoamine oxidase activities toward different substrates: effects in rat brain of chronic administration of manganese chloride and ageing. J Neurochem 36: 2037–2043
Levitt P, Pintar J, Breakfield X (1982) Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad USA 79: 6385–6389
Mandybur TT, Ormsby I, Zemian FP (1989) Cerebral aging: a quantitative study of gliosis in old nude mice. Acta Neuropathol 77: 507–513
Matsumura A, Higuchi K, Shimizu K, Hosokawa M, Hashimoto K, Yasuhira K, Takeda T (1982) A novel amyloid fibril protein isolated from senescence-accelerated mice. Lab Invest 47: 270–275
Matsushita M, Tsuboyama T, Kasai R, Okumura H, Yamamuro T, Higuchi K, Kohno A, Yonezu T, Utani A, Umezawa M, Takeda T (1986) Age-related changes in bone mass in the senecence accelerated mouse (SAM): SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol 125: 276–283
Miyamoto M, Kiyota Y, Yamazaki N, Nagaoka A, Matsuo T, Nagawa Y, Takeda T (1986) Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol Behav 38: 399–406
Naiki H, Higuchi K, Yonezu T, Hosokawa M, Takeda T (1988) Metabolism of senile amyloid precursor and amyloidogenesis: age-related acceleration of apolipoprotein A-II clearance in the senescence accelerated mouse. Am J Pathol 130: 579–587
Nakamura S, Kawamata T, Akiguchi I, Kameyama M, Nakamura N, Kimura H (1990) Expression of monoamine oxidase activity in astrocytes of senile plaques. Acta Neuropathol 80: 419–425
Nakamura S, Kawamata T, Yasuhara O, Takemura M, Akiguchi I, Kimura J, Kimura T (1991) The histochemical demonstration of monoamine oxidase-containing neurons in the human hypothalamus. Neuroscience 44: 457–463
Nakamura S, Akiguchi I, Kimura J (1993) A subpopulation of mouse striatal cholinergic neurons show monoamine oxidase activity. Neurosci Lett 161: 141–144
Nomura Y, Wang B-X, Qi S-B, Namba T, Kaneko S (1989) Biochemical changes related to aging in the senescence-accelerated mouse. Exp Gerontol 24: 49–55
O'Carroll A-M, Fowler CJ, Philips JP, Tobbia I, Tipton KF (1983) The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn-Schmiedebergs Arch Pharmacol 322: 198–202
Ohta A, Hirano T, Yagi H, Tanaka S, Hosokawa M, Takeda T (1989) Behavioral characteristics of the SAM-P/8 strain in Sidman active avoidance task. Brain Res 498: 195–198
Oreland L, Gottfries CG (1986) Brain and brain monoamine oxidase in aging and dementia of Alzheimer's type. Prog Neuropsychopharmacol Biol Psychiatry 10: 533–540
Riederer P, Jellinger K (1983) Neurochemical insights into monoamine oxidase inhibitors, with special reference to deprenyl (selegiline). Acta Neuroal Scand [Suppl] 95: 43–55
Saura J, Richard JG; Mahy N (1994) Differential age-related changes of MAO-A and MAO-B in mouse brain and peripheral organs. Neurobiol Aging 15: 105–114
Squires RF (1972) Multiple forms of monoamine oxidase in intact mitochondria as characterized by selective inhibitors and thermal stability: a comparison of eight mammalian species. Adv Biochem Psychopharmacol 5: 355–370
Strolin-Benedetti M, Dostert P (1989) Monoamine oxidase, brain aging and degenerative disease. Biochem Pharmacol 38: 555–561
Strolin-Benedetti M, Keane PE (1980) Differential changes in monoamine oxidase A and B activity in the aging rat brain. J Neurochem 35: 1026–1032
Sugiyama H, Hainfellner JA, Lassmann H, Indravasu S, Budka H (1993) Uncommon types of polyglucosan bodies in the human brain: distribution and relation to disease. Acta Neuropathol 86: 484–490
Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T (1981) A new murine model of accelerated senescence. Mech Ageing Dev 17: 183–194
Takeda T, Hosokawa M, Higuchi K (1991) Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. J Am Geriatr Soc 329: 911–919
Takemura M, Nakamura S, Akiguchi I, Ueno M, Oka N, Ishikawa S, Shimada A, Kimura J, Takeda T (1993) β/A4 proteinlike immunoreactive granular structures in the brain of senescence-accelerated mouse. Am J Pathol 142: 1887–1897
Westlund KN, Denney RM, Kochersberger LM, Rose RM, Abell CW (1985) Distinct monoamine oxidase A and B populations in primate brain. Science 230: 181–183
Westlund KN, Denney RM, Rose RM, Abell CW (1988) Localization of distinct monoamine oxdiase A and monoamine oxidase B cell populations in human brain stem. Neuroscience 25: 439–456
Yagi H, Katoh S, Akiguchi I, Takeda T (1988) Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res 474: 86–93
Yagi H, Irino M, Matsushita T, Katoh S, Umezawa M, Tsuboyama T, Hosokawa M, Akiguchi I, Tokunaga R, Takeda T (1989) Spontaneous spongy degeneration of the brain stem in SAM-P/8 mice, a newly developed memory-deficient strain. J Neuropathol Exp Neurol 48: 577–590
Yonezu T, Tsunasawa S, Higuchi K, Kogishi K, Naiki H, Hanada K, Sakiyama F, Takeda T (1987) A molecular pathologic approach to murine senile amyloidosis: serum precursor-apolipoprotein A-II variant (Pro5-Gln) presents only in the senile amyloidosis-prone SAM-P/1 and SAM P/2 mice. Lab Invest 57: 65–70
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nakamura, S., Akiguchi, I., Seriu, N. et al. Monoamine oxidase-B-positive granular structures in the hippocampus of aged senescence-accelerated mouse (SAMP8). Acta Neuropathol 90, 626–632 (1995). https://doi.org/10.1007/BF00318576
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318576