Abstract
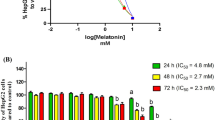
4-Hydroxycoumarin is an aromatic substance which is metabolized in liver and used as a therapeutic agent for various diseases. We aimed to determine the impact of 4-Hydroxycoumarin on HepG2 cells according to their viability, proliferation, adhesion and gene expression of cellular behavior parameters. Inhibitory concentration 50 (IC50) of 4-Hydroxycoumarin was detected on HepG2 cells. After determining the optimal time and concentration, the effect of 4-Hydroxycoumarin on viability, proliferation and adhesion of HepG2 cells were observed. Gene expressions of Ki-67, MMP-2, MMP-9 and piR-823 expression were determined by using Real Time-Polymerase Chain Reaction. IC50 value of 4-Hydroxycoumarin on HepG2 cells was 5 μM at the 48 h (p < 0.001). 5 μM at the 48 h of 4-Hydroxycoumarin caused to decrease of proliferation (p < 0.001) and viability of HepG2 cells (p < 0.001). Viability rate were supported by hematoxylin-eosin staining. Adhesion of cells increased on 4-Hydroxycoumarin treated cells compared to control (p < 0.001). While Ki-67 gene expression of 4-Hydroxycoumarin treated group decreased (p < 0.001); upregulation of MMP-2, MMP-9 and piR-823 expressions were determined in 4-Hydroxycoumarin treated group (p < 0.001). According to the cellular and genetic perspective, 4-Hydroxycoumarin might be useful to treat hepatocellular carcinoma. High adhesion and proliferation are the main characteristics of HepG2 cells, 4-Hydroxycoumarin treatment caused to lose these functions. The genetic markers of these characteristics also supported the same result. These are first findings about the effect of 4-Hydroxycoumarin on piR-823 and genes which are key features of cellular survival mechanisms.




Similar content being viewed by others
REFERENCES
Abraham, K., Wohrlin, F., Lindtner, O., et al., Toxicology and risk assessment of coumarin: Focus on human data, Mol. Nutr. Food Res., 2010, vol. 54, no. 2, pp. 228–239. https://doi.org/10.1002/mnfr.200900281
Bar-Ziv, R., Tlusty, T., Moses, E., et al., Pearling in cells: A clue to understanding cell shape, Proc. Natl. Acad. Sci. U. S. A., 1999, vol. 96, no. 18, pp. 10140–10145. https://doi.org/10.1073/pnas.96.18.10140
Breckenridge, A.M., Leck, J.B., Park, B.K., et al., J. Pharmacol., 1978, vol. 64, art. ID 399.
Bullwinkel, J., Baron-Luhr, B., Ludemann, A., et al., Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells, J. Cell. Physiol., 2006, vol. 206, no. 3, pp. 624–635. https://doi.org/10.1002/jcp.20494
Chalbatani, G.M., Dana, H., Memari, F., et al., Biological function and molecular mechanism of piRNA in cancer, Pract. Lab. Med., 2019, vol. 13, art. ID e00113. https://doi.org/10.1016/j.plabm.2018.e00113
Chen, W.C., Liu, L., Shen, Y.F., et al., A new coumarin derivative plays a role in rhabdoviral clearance by interfering glycoprotein function during the early stage of viral infection, Cell. Signaling, 2018, vol. 51, pp. 199–210. https://doi.org/10.1016/j.cellsig.2018.08.007
Cheng, J., Deng, H.X., Xiao, B.X., et al., piR-823, a novel non-coding small RNA, demonstrates in vitro and in vivo tumor suppressive activity in human gastric cancer cells, Cancer Lett., 2012, vol. 315, pp. 12–17. https://doi.org/10.1016/j.canlet.2011.10.004
Chien, Y.C., Liu, L.C., Ye, H.Y., et al., EZH2 promotes migration and invasion of triple-negative breast cancer cells via regulating TIMP2-MMP-2/-9 pathway, Am. J. Cancer Res., 2018, vol. 8, no. 3, pp. 422–434.
Cui, J., Dong, B.W., Liang, P., et al., Effect of c-myc, Ki-67, MMP-2 and VEGF expression on prognosis of hepatocellular carcinoma patients undergoing tumor resection, World J. Gastroenterol., 2004, vol. 10, pp. 1533–1536. https://doi.org/10.3748/wjg.v10.i10.1533
Cui, L., Lou, Y., Zhang, X., et al., Detection of circulating tumor cells in peripheral blood from patients with gastric cancer using piRNAs as markers, Clin. Biochem., 2011, vol. 44, no. 13, pp. 1050–1057. https://doi.org/10.1016/j.clinbiochem.2011.06.004
Dehn, P.F., White, C.M., Conners, D.E., et al., Characterization of the human hepatocellular carcinoma (hepg2) cell line as an in vitro model for cadmium toxicity studies, In Vitro Cell. Dev. Biol.: Anim., 2004, vol. 40, nos. 5–6, pp. 172–182. https://doi.org/10.1290/1543-706X(2004)40<172:COTHHC>2.0.CO;2
Deryugina, E.I. and Quigley, J.P., Matrix metalloproteinases and tumor metastasis, Cancer Metastasis Rev., 2006, vol. 25, pp. 9–34. https://doi.org/10.1007/s10555-006-7886-9
Dowsett, M., Nielsen, T.O., A’hern, R., et al., Assessment of Ki67 in breast cancer: Updated recommendations from the International Ki67 in Breast Cancer working group, J. Natl. Cancer Inst., 2011, vol. 103, no. 7, pp. 1656–1664. https://doi.org/10.1093/jnci/djr393
Egan, D., James, P., Cooke, D., et al., Studies on the cytostatic and cytotoxic effects and mode of action of 8-nitro-7-hydroxycoumarin, Cancer Lett., 1997, vol. 118, no. 2, pp. 201–211. https://doi.org/10.1016/s0304-3835(97)00331-5
El-Serag, H.B. and Rudolph, K.L., Hepatocellular carcinoma: epidemiology and molecular carcinogenesis, Gastroenterology, 2007, vol. 132, no. 7, pp. 2557–2576. https://doi.org/10.1053/j.gastro.2007.04.061
Emami, S. and Dadashpour, S., Current developments of coumarin-based anti-cancer agents in medicinal chemistry, Eur. J. Med. Chem., 2015, vol. 102, pp. 611–630. https://doi.org/10.1016/j.ejmech.2015.08.033
Finn, G.J., Creaven, B., and Egan, D.A., Study of the in vitro cytotoxic potential of natural and synthetic coumarin derivatives using human normal and neoplastic skin cell lines, Melanoma Res., 2001, vol. 11, pp. 461–467.
Funaya, C., Antony, C., Sachidanandam, R., et al., Miwi catalysis is required for piRNA amplification‑independent LINE1 transposon silencing, Nature, 2011, vol. 480, pp. 264–267.
Gu, J., Li, Y., Fan, L., et al., Identification of aberrantly expressed long non-coding RNAs in stomach adenocarcinoma, Oncotarget, 2017, vol. 8, pp. 49201–49216. https://doi.org/10.18632/oncotarget.17329
Han, H., Zhang, Z.-F., Zhang, J.-F., et al., Novel coumarin derivatives: Synthesis, anti-breast cancer activity and docking study, Main Group Chem., 2019, vol. 18, pp. 71–79. https://doi.org/10.3233/mgc-180682
Iliev, R., Stanik, M., Fedorko, M., et al., Decreased expression levels of PIWIL1, PIWIL2, and PIWIL4 are associated with worse survival in renal cell carcinoma patients, OncoTargets Ther., 2016, vol. 9, pp. 217–222. https://doi.org/10.2147/OTT.S91295
Ingber, D.E., Tensegrity II. How structural networks influence cellular information processing networks, J. Cell Sci., 2003, vol. 116, no. 8, pp. 1397–1408. https://doi.org/10.1242/jcs.00360
Jacobs, D.I., Qin, Q., Lerro, M.C., et al., PIWI-interacting RNAs in gliomagenesis: evidence from post-GWAS and functional analyses, Cancer Epidemiol., Biomarkers Prev., 2016, vol. 25, no. 7, pp. 1073–1080. https://doi.org/10.1158/1055-9965.EPI-16-0047
Jimenez-Orozco, F.A., Lopez-Gonzalez, J.S., Nieto-Rodriguez, A., et al., Decrease of cyclin D1 in the human lung adenocarcinoma cell line A-427 by 7-hydroxycoumarin, Lung Cancer, 2001, vol. 34, no. 2, pp. 185–194.
Zanker, K.S., Blumel, J., Lange, J.R., et al., Coumarin in melanoma patients: an experimental and clinical study, Drugs Exp. Clin. Res., 1984, vol. 10, pp. 767–774.
Kawaii, S., Tomono, Y., Ogawa, K., et al., The antiproliferative effect of coumarins on several cancer cell lines, Anticancer Res., 2001, vol. 21, pp. 917–923.
Keating, J.G. and O’kennedy, R., The Chemistry and Occurrence of Coumarins, New York: Wiley, 1997.
King, K.L., Hwang, J.J., Chau, G.Y., et al., Ki-67 expression as a prognostic marker in patients with hepatocellular carcinoma, J. Gastroenterol. Hepatol., 1998, vol. 13, pp. 273–279. https://doi.org/10.1111/j.1440-1746.1998.01555.x
Kokron, O., Maca, S., Gasser, G., et al., Cimetidine and coumarin therapy of renal-cell carcinoma, Oncology, 1991, vol. 48, pp. 102–126.
Lake, B.G., Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment, Food Chem. Toxicol., 1999, vol. 37, pp. 423–453.
Li, J., Xue, X.Y., Li, X., et al., Synthesis of biscoumarin and dihydropyran derivatives as two novel classes of potential anti-bacterial derivatives, Arch. Pharmacal Res., 2016, vol. 39, pp. 1349–1355. https://doi.org/10.1007/s12272-015-0614-7
Lirdprapamongkol, K., Kramb, J.P., Suthiphongchai, T., et al., Vanillin suppresses metastatic potential of human cancer cells through PI3K inhibition and decreases angiogenesis in vivo, J. Agric. Food Chem., 2009, vol. 57, pp. 3055–3063. https://doi.org/10.1021/jf803366f
Liu, Y., Dou, M., Song, X., et al., The emerging role of the piRNA/piwi complex in cancer, Mol. Cancer, 2019, vol. 18, art. ID 123. https://doi.org/10.1186/s12943-019-1052-9
Lopez-Gonzalez, J.S., Prado-Garcia, H., Aguilar-Cazares, D., et al., Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines, Lung Cancer, 2004, vol. 43, pp. 275–283. https://doi.org/10.1016/j.lungcan.2003.09.005
Man, S.L., Gao, W.Y., Zhang, Y.J., et al., Formosanin C‑inhibited pulmonary metastasis through repression of matrix metalloproteinases on mouse lung adenocarcinoma, Cancer Biol. Ther., 2011, vol. 11, pp. 592–598. https://doi.org/10.4161/cbt.11.6.14668
Marshall, M.E., Butler, K., and Fried, A., Phase I evaluation of coumarin (1,2-benzopyrone) and cimetidine in patients with advanced malignancies, Mol. Biother., 1991, vol. 3, no. 3, pp. 170–178.
Marshall, M.E., Kervin, K., Benefield, C., et al., Growth-inhibitory effects of coumarin (1,2-benzopyrone) and 7-hydroxycoumarin on human malignant cell lines in vitro, J. Cancer Res. Clin. Oncol., 1994, vol. 120, pp. S3–S10.
Marusyk, A., Tabassum, D.P., Altrock, P.M., et al., Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity, Nature, 2014, vol. 514, pp. 54–58. https://doi.org/10.1038/nature13556
Mohler, J.L., Gomella, L.G., Crawford, E.D., et al., Phase II evaluation of coumarin (1,2-benzopyrone) in metastatic prostatic carcinoma, Prostate, 1992, vol. 20, no. 2, pp. 123–131.
Mohler, J.L., Williams, B.T., Thompson, I.M., et al., Coumarin (1,2-benzopyrone) for the treatment of prostatic carcinoma, J. Cancer Res. Clin. Oncol., 1994, vol. 120, pp. S35–S38.
Mosmann, T., Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, vol. 65, nos. 1–2, pp. 55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Nasr, T., Bondock, S., and Youns, M., Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives, Eur. J. Med. Chem., 2014, vol. 76, pp. 539–548. https://doi.org/10.1016/j.ejmech.2014.02.026
Öner, C., Turgut Coşan, D., and Çolak, E., Estrogen and androgen hormone levels modulate the expression of PIWI interacting RNA in prostate and breast cancer, PLoS One, 2016, vol. 11, art. ID e0159044. https://doi.org/10.1371/journal.pone.0159044
Ozata, D.M., Gainetdinov, I., Zoch, A., et al., PIWI-interacting RNAs: small RNAs with big functions, Nat. Rev. Genet., 2019, vol. 20, pp. 89–108. https://doi.org/10.1038/s41576-018-0073-3
Phillips, C., Zeringue, A.L., Mcdonald, J.R., et al., Tumor necrosis factor inhibition and head and neck cancer recurrence and death in rheumatoid arthritis, PLoS One, 2015, vol. 10, art. ID e0143286. https://doi.org/10.1371/journal.pone.0143286
Rennenberg, R.J., Van Varik, B.J., Schurgers, L.J., et al., Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans, Blood, 2010, vol. 115, no. 24, pp. 5121–5123. https://doi.org/10.1182/blood-2010-01-264598
Salinas-Jazmin, N., De La Fuente, M., Jaimez, R., et al., Antimetastatic, antineoplastic, and toxic effects of 4-hydroxycoumarin in a preclinical mouse melanoma model, Cancer Chemother. Pharmacol., 2010, vol. 65, pp. 931–940. https://doi.org/10.1007/s00280-009-1100-z
Shokoohinia, Y., Hosseinzadeh, L., Alipour, M., et al., Comparative evaluation of cytotoxic and apoptogenic effects of several coumarins on human cancer cell lines: Osthole induces apoptosis in p53-deficient H1299 cells, Adv. Pharmacol. Sci. 2014, art. ID 847574.
Sobecki, M., Mrouj, K., Colinge, J., et al., Cell-Cycle regulation accounts for variability in Ki-67 expression levels, Cancer Res., 2017, vol. 77, no. 10, pp. 2722–2734. https://doi.org/10.1158/0008-5472.CAN-16-0707
Stefanova, T.H., Nikolova, N.J., Toshkova, R.A., et al., Antitumor and immunomodulatory effect of coumarin and 7-hydroxycoumarin against Sarcoma 180 in mice, J. Exp. Ther. Oncol., 2007, vol. 6, no. 2, pp. 107–115.
Thornes, R.D., Daly, L., Lynch, G., et al., Treatment with coumarin to prevent or delay recurrence of malignant melanoma, J. Cancer Res. Clin. Oncol., 1994, vol. 120, pp. S32–S34.
Ujjin, P., Satarug, S., Vanavanitkun, Y., et al., Variation in coumarin 7-hydroxylase activity associated with genetic polymorphism of cytochrome P450 2A6 and the body status of iron stores in adult Thai males and females, Pharmacogenetics, 2002, vol. 12, no. 3 pp. 241–249.
Vázquez, R., Riveiro, M.E., Vermeulen, M., et al., Structure-anti-leukemic activity relationship study of ortho-dihydroxycoumarins in U-937 cells: key role of the δ-lactone ring in determining differentiation-inducing potency and selective pro-apoptotic action, Bioorg. Med. Chem., 2012, vol. 20, pp. 5537–5549.
Velasco-Velazquez, M.A., Agramonte-Hevia, J., Barrera, D., et al., 4-Hydroxycoumarin disorganizes the actin cytoskeleton in B16–F10 melanoma cells but not in B82 fibroblasts, decreasing their adhesion to extracellular matrix proteins and motility, Cancer Lett., 2003, vol. 198, pp. 179–186. https://doi.org/10.1016/S0304-3835(03)00333-1
Velasco-Velázquez, M.A., Salinas-Jazmín, N., Mendoza-Patiño, N., et al., Reduced paxillin expression contributes to the antimetastatic effect of 4-hydroxycoumarin on B16-F10 melanoma cells, Cancer Cell. Int., 2008, art. ID 8.
Vianna, D.R., Hamerski, L., Figueiro, F., et al., Selective cytotoxicity and apoptosis induction in glioma cell lines by 5-oxygenated-6,7-methylenedioxycoumarins from Pterocaulon species, Eur. J. Med. Chem., 2012, vol. 57, pp. 268–274. https://doi.org/10.1016/j.ejmech.2012.09.007
Wu, X.Q., Huang, C., Jia, Y.M., et al., Novel coumarin-dihydropyrazole thio-ethanone derivatives: Design, synthesis and anticancer activity, Eur. J. Med. Chem., 2014, vol. 74, pp. 717–725. https://doi.org/10.1016/j.ejmech.2013.06.014
Xiao, Z., Shen, J., Zhang, L., et al., Therapeutic targeting of noncoding RNAs in hepatocellular carcinoma: Recent progress and future prospects (Review), Oncol. Lett., 2018, vol. 15, pp. 3395–3402. https://doi.org/10.3892/ol.2018.7758
Xu, C., Rao, Y.S., Xu, B., et al., An in vivo pilot study characterizing the new CYP2A6*7, *8, and *10 alleles, Biochem Biophys. Res. Commun., 2002, vol. 290, no. 1, pp. 318–324. https://doi.org/10.1006/bbrc.2001.6209
Yamashiro, H. and Siomi, M.C., PIWI-interacting RNA in Drosophila: Biogenesis, transposon regulation, and beyond., Chem. Rev., 2018, vol. 118, no. 8, pp. 4404–4421. https://doi.org/10.1021/acs.chemrev.7b00393
Yan, H., Wu, Q.L., Sun, C.Y., et al., piRNA-823 contributes to tumorigenesis by regulating de novo DNA methylation and angiogenesis in multiple myeloma, Leukemia, 2015, vol. 29, pp. 196–206. https://doi.org/10.1038/leu.2014.135
Yang, Y., Lu, N., Zhou, J., et al., Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis, Rheumatology (Oxford), 2008, vol. 47, no. 9, pp. 1299–1310. https://doi.org/10.1093/rheumatology/ken225
Yin, J., Jiang, X.Y., Qi, W., et al., piR-823 contributes to colorectal tumorigenesis by enhancing the transcriptional activity of HSF1, Cancer Sci., 2017, vol. 108, no. 9, pp. 1746–1756. https://doi.org/10.1111/cas.13300
Zhang, J., Zhang, D., Wu, G-Q., et al., Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression, Hepatobiliary Pancreatic Dis. Int., 2013, vol. 12, pp. 305–309. https://doi.org/10.1016/s1499-3872(13)60048-x
Funding
This study is funded by 2209-A Programme of the Scientific and Technological Research Council of Turkey (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
AUTHORS CONTRIBUTION
All authors (Çağri Öner (ÇÖ), Dilara Soyergin (DS), Ahmet Özyurt (AÖ), Ertuğrul ÇOLAK (EÇ)) substantially contributed to the conception and design of the study, the acquisition of data, including conducting the experiments and analysis and interpretation of the results. They took part in interpreting the results statistically. Furthermore, they drafted the manuscript and critically revised it, approved the final version, and took responsibility for the statements made in the article.
About this article
Cite this article
Öner, Ç., Soyergin, D., Özyurt, A. et al. 4-Hydroxycoumarin Effects on Both Cellular and Genetic Characteristics of Hepatocellular Carcinoma Cells. Cytol. Genet. 56, 292–300 (2022). https://doi.org/10.3103/S0095452722030094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452722030094