Abstract
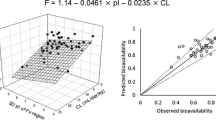
The prediction of bioavailability is one of the major barriers in the clinical translation of subcutaneously (SC) administered therapeutic monoclonal antibodies (mAbs) due to the lack of reliable in vitro and preclinical in vivo predictive models. Recently, multiple linear regression (MLR) models were developed to predict human SC bioavailability of mAbs using human linear clearance (CL) and isoelectric point (pI) of the whole antibody or Fv regions as independent variables. Unfortunately, these models cannot be applied to mAbs at the preclinical development stage because human CLs of these mAbs are unknown. In this study, we predicted human SC bioavailability of mAbs using preclinical data only by two approaches. In the first approach, allometric scaling was used to predict human linear CL from non-human primate (NHP) linear CL. The predicted human CL and the pI of the whole antibody or Fv regions were then incorporated into two previously published MLR models to predict the human bioavailability of 61 mAbs. In the second approach, two MLR models were developed using NHP linear CL and the pI of whole antibody or Fv regions of 41 mAbs in a training set. The two models were validated using an independent test dataset containing 20 mAbs. The four MLR models generated 77–85% of predictions within 0.8- to 1.2-fold deviations from observed human bioavailability. Overall, this study demonstrated that human SC bioavailability of mAbs at the preclinical stage could be predicted using NHP CL and pI of mAbs.
Graphical Abstract







Similar content being viewed by others
Data Availability
The author confirms that the data supporting findings of this study are available within the article and supplementary materials.
Abbreviations
- 3D_pI:
-
3D isoelectric point
- AUC0–tau :
-
Area under the plasma concentration–time curve from time zero to the end of the dosing interval
- BW:
-
Body weight
- CL:
-
Clearance
- EMA:
-
European Medicines Agency
- ExPASy:
-
Expert protein analysis system
- Fab:
-
Antigen-binding fragment
- FDA:
-
Food and Drug Administration
- Fv:
-
Variable fragment
- GMFE:
-
Geometric mean fold error
- IV:
-
Intravenous
- mAb:
-
Monoclonal antibody
- MLR:
-
Multiple linear regression
- NHP:
-
Non-human primate
- PK:
-
Pharmacokinetics
- pI:
-
Isoelectric point
- PwFE:
-
Prediction within 0.8- to 1.2-fold errors
- SC:
-
Subcutaneous
References
Collins DS, Sanchez-Felix M, Badkar AV, Mrsny R. Accelerating the development of novel technologies and tools for the subcutaneous delivery of biotherapeutics. J Control Release. 2020;321:475–82. https://doi.org/10.1016/j.jconrel.2020.02.036.
Bown HK, Bonn C, Yohe S, Yadav DB, Patapoff TW, Daugherty A, et al. In vitro model for predicting bioavailability of subcutaneously injected monoclonal antibodies. J Control Release. 2018;273:13–20. https://doi.org/10.1016/j.jconrel.2018.01.015.
Lou H, Hageman MJ. Machine learning attempts for predicting human subcutaneous bioavailability of monoclonal antibodies. Pharm Res. 2021;38(3):451–60. https://doi.org/10.1007/s11095-021-03022-y.
Haraya K, Tachibana T. Estimation of clearance and bioavailability of therapeutic monoclonal antibodies from only subcutaneous injection data in humans based on comprehensive analysis of clinical data. Clin Pharmacokinet. 2021;60(10):1325–34. https://doi.org/10.1007/s40262-021-01023-z.
Zou P. Predicting human bioavailability of subcutaneously administered fusion proteins and monoclonal antibodies using human intravenous clearance or antibody isoelectric point. AAPS J. 2023;25(3):31. https://doi.org/10.1208/s12248-023-00798-2.
Menochet K, Yu H, Wang B, Tibbitts J, Hsu CP, Kamath AV, et al. Non-human primates in the PKPD evaluation of biologics: needs and options to reduce, refine, and replace. A BioSafe White Paper MAbs. 2022;14(1):2145997. https://doi.org/10.1080/19420862.2022.2145997.
Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol. 2009;49(12):1382–402. https://doi.org/10.1177/0091270009337134.
Oitate M, Masubuchi N, Ito T, Yabe Y, Karibe T, Aoki T, et al. Prediction of human pharmacokinetics of therapeutic monoclonal antibodies from simple allometry of monkey data. Drug Metab Pharmacokinet. 2011;26(4):423–30. https://doi.org/10.2133/dmpk.dmpk-11-rg-011.
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3(1):61–6. https://doi.org/10.4161/mabs.3.1.13799.
Haraya K, Tachibana T, Nezu J. Quantitative prediction of therapeutic antibody pharmacokinetics after intravenous and subcutaneous injection in human. Drug Metab Pharmacokinet. 2017;32(4):208–17. https://doi.org/10.1016/j.dmpk.2017.05.002.
Sanchez-Felix M, Burke M, Chen HH, Patterson C, Mittal S. Predicting bioavailability of monoclonal antibodies after subcutaneous administration: open innovation challenge. Adv Drug Deliv Rev. 2020;167:66–77. https://doi.org/10.1016/j.addr.2020.05.009.
Liu S, Verma A, Kettenberger H, Richter WF, Shah DK. Effect of variable domain charge on in vitro and in vivo disposition of monoclonal antibodies. MAbs. 2021;13(1):1993769. https://doi.org/10.1080/19420862.2021.1993769.
Zheng F, Hou P, Corpstein CD, Park K, Li T. Multiscale pharmacokinetic modeling of systemic exposure of subcutaneously injected biotherapeutics. J Control Release. 2021;337:407–16. https://doi.org/10.1016/j.jconrel.2021.07.043.
Grevys A, Frick R, Mester S, Flem-Karlsen K, Nilsen J, Foss S, et al. Antibody variable sequences have a pronounced effect on cellular transport and plasma half-life. iScience. 2022;25(2):103746. https://doi.org/10.1016/j.isci.2022.103746.
Ahmed L, Gupta P, Martin KP, Scheer JM, Nixon AE, Kumar S. Intrinsic physicochemical profile of marketed antibody-based biotherapeutics. Proc Natl Acad Sci U S A. 2021;118(37). https://doi.org/10.1073/pnas.2020577118.
Zou P, Wang F, Wang J, Lu Y, Tran D, Seo SK. Impact of injection sites on clinical pharmacokinetics of subcutaneously administered peptides and proteins. J Control Release. 2021;336:310–21. https://doi.org/10.1016/j.jconrel.2021.06.038.
Author information
Authors and Affiliations
Contributions
PZ designed the work, collected the data, conducted the analysis, and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
PZ is a current employee of Daiichi Sankyo Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, P. Predicting Human Bioavailability of Subcutaneously Administered Monoclonal Antibodies Using Non-human Primate Linear Clearance and Antibody Isoelectric Point. AAPS J 25, 53 (2023). https://doi.org/10.1208/s12248-023-00818-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00818-1