Abstract
Background and objective
The subcutaneous injection of therapeutic monoclonal antibodies is increasingly used in the treatment of several diseases because of its convenience. Thus, a simple and accurate method of predicting the pharmacokinetics of monoclonal antibodies after a subcutaneous injection in humans would be a valuable tool for preclinical/clinical development. In this study, we investigated whether the pharmacokinetics of monoclonal antibodies after a subcutaneous injection in humans can be predicted using only pharmacokinetic data after a subcutaneous injection in cynomolgus monkeys.
Methods
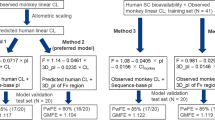
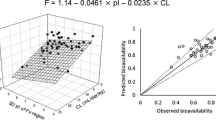
First, we compared the accuracy of three approaches to predict the apparent clearance (CL/F) and apparent volume of distribution (Vd/F) for 15 monoclonal antibodies in humans (1) allometric scaling from cynomolgus monkeys; (2) geometric mean of reported values in humans; (3) estimation from a regression line based on CL/F in humans [only Vd/F]). Then, using the predicted CL/F and Vd/F, and the geometric mean of reported absorption rate constant of mAbs the plasma concentration–time profiles of 13 monoclonal antibodies after subcutaneous injections in humans were simulated.
Results
In a comparison of approaches, the first approach showed the best prediction accuracy for CL/F with an exponent of 0.9 (100% and 73% prediction accuracy within 2- and 1.5-fold of the observed value),and the third approach was the best for Vd/F (100% prediction accuracy within 1.5-fold of the observed value). Next, using the first approach for CL/F and the third approach for Vd/F, we accurately predicted the plasma concentration–time profiles of 13 monoclonal antibodies after subcutaneous injections in humans.
Conclusion
This simple approach can be applied in preclinical and clinical settings to predict the pharmacokinetics of monoclonal antibodies after subcutaneous injections in humans. Further, this approach requires only CL/F after a subcutaneous injection in cynomolgus monkeys, contributing to animal welfare and reducing costs.






Similar content being viewed by others
References
Tsumoto K, Isozaki Y, Yagami H, Tomita M. Future perspectives of therapeutic monoclonal antibodies. Immunotherapy. 2019;11(2):119–27. https://doi.org/10.2217/imt-2018-0130.
Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. mAbs. 2015;7(1):9–14. https://doi.org/10.4161/19420862.2015.989042.
Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–88. https://doi.org/10.1002/psp4.12224.
Viola M, Sequeira J, Seica R, Veiga F, Serra J, Santos AC, et al. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release. 2018;286:301–14. https://doi.org/10.1016/j.jconrel.2018.08.001.
Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol. 2010;28(11):1203–7. https://doi.org/10.1038/nbt.1691.
Igawa T, Maeda A, Haraya K, Tachibana T, Iwayanagi Y, Mimoto F, et al. Engineered monoclonal antibody with novel antigen-sweeping activity in vivo. PLoS ONE. 2013;8(5):e63236. https://doi.org/10.1371/journal.pone.0063236.
Haraya K, Tachibana T, Iwayanagi Y, Maeda A, Ozeki K, Nezu J, et al. PK/PD analysis of a novel pH-dependent antigen-binding antibody using a dynamic antibody-antigen binding model. Drug Metab Pharmacokinet. 2016;31(2):123–32. https://doi.org/10.1016/j.dmpk.2015.12.007.
Igawa T, Haraya K, Hattori K. Sweeping antibody as a novel therapeutic antibody modality capable of eliminating soluble antigens from circulation. Immunol Rev. 2016;270(1):132–51. https://doi.org/10.1111/imr.12392.
Shpilberg O, Jackisch C. Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br J Cancer. 2013;109(6):1556–611. https://doi.org/10.1038/bjc.2013.371.
Haraya K, Tachibana T, Igawa T. Improvement of pharmacokinetic properties of therapeutic antibodies by antibody engineering. Drug Metab Pharmacokinet. 2019;34(1):25–41. https://doi.org/10.1016/j.dmpk.2018.10.003.
Iwasaki K, Uno Y, Utoh M, Yamazaki H. Importance of cynomolgus monkeys in development of monoclonal antibody drugs. Drug Metab Pharmacokinet. 2019;34(1):55–63. https://doi.org/10.1016/j.dmpk.2018.02.003.
Haraya K, Tachibana T, Nezu J. Quantitative prediction of therapeutic antibody pharmacokinetics after intravenous and subcutaneous injection in human. Drug Metab Pharmacokinet. 2017;32(4):208–17. https://doi.org/10.1016/j.dmpk.2017.05.002.
Oitate M, Nakayama S, Ito T, Kurihara A, Okudaira N, Izumi T. Prediction of human plasma concentration-time profiles of monoclonal antibodies from monkey data by a species-invariant time method. Drug Metab Pharmacokinet. 2012;27(3):354–9. https://doi.org/10.2133/dmpk.dmpk-11-sh-059.
Nnane IP, Han C, Jiao Q, Tam SH, Davis HM, Xu Z. Modification of the Fc region of a human anti-oncostatin M monoclonal antibody for higher affinity to FcRn receptor and extension of half-life in cynomolgus monkeys. Basic Clin Pharmacol Toxicol. 2017;121(1):13–211. https://doi.org/10.1111/bcpt.12761.
Haraya K, Tachibana T, Nezu J. Predicting pharmacokinetic profile of therapeutic antibodies after iv injection from only the data after sc injection in cynomolgus monkey. Xenobiotica. 2017;47(3):194–201. https://doi.org/10.1080/00498254.2016.1174792.
Oh CK, Faggioni R, Jin F, Roskos LK, Wang B, Birrell C, et al. An open-label, single-dose bioavailability study of the pharmacokinetics of CAT-354 after subcutaneous and intravenous administration in healthy males. Br J Clin Pharmacol. 2010;69(6):645–55. https://doi.org/10.1111/j.1365-2125.2010.03647.x.
Struemper H, Thapar M, Roth D. Population pharmacokinetic and pharmacodynamic analysis of belimumab administered subcutaneously in healthy volunteers and patients with systemic lupus erythematosus. Clin Pharmacokinet. 2018;57(6):717–28. https://doi.org/10.1007/s40262-017-0586-5.
Oitate M, Masubuchi N, Ito T, Yabe Y, Karibe T, Aoki T, et al. Prediction of human pharmacokinetics of therapeutic monoclonal antibodies from simple allometry of monkey data. Drug Metabol Pharmacokinet. 2011;26(4):423–30.
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned. mAbs. 2011;3(1):61–6. https://doi.org/10.4161/mabs.3.1.13799.
Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, et al. Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet. 2011;50(2):131–42. https://doi.org/10.2165/11537430-000000000-00000.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–70. https://doi.org/10.1208/s12248-012-9367-0.
Luu KT, Bergqvist S, Chen E, Hu-Lowe D, Kraynov E. A model-based approach to predicting the human pharmacokinetics of a monoclonal antibody exhibiting target-mediated drug disposition. J Pharmacol Exp Ther. 2012;341(3):702–8. https://doi.org/10.1124/jpet.112.191999.
Wiczling P, Rosenzweig M, Vaickus L, Jusko WJ. Pharmacokinetics and pharmacodynamics of a chimeric/humanized anti-CD3 monoclonal antibody, otelixizumab (TRX4), in subjects with psoriasis and with type 1 diabetes mellitus. J Clin Pharmacol. 2010;50(5):494–506. https://doi.org/10.1177/0091270009349376.
Yan X, Mager DE, Krzyzanski W. Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2010;37(1):25–47. https://doi.org/10.1007/s10928-009-9142-8.
Zheng Y, Tesar DB, Benincosa L, Birnbock H, Boswell CA, Bumbaca D, et al. Minipig as a potential translatable model for monoclonal antibody pharmacokinetics after intravenous and subcutaneous administration. mAbs. 2012;4(2):243–55. https://doi.org/10.4161/mabs.4.2.19387.
Bown HK, Bonn C, Yohe S, Yadav DB, Patapoff TW, Daugherty A, et al. In vitro model for predicting bioavailability of subcutaneously injected monoclonal antibodies. J Controlled Release. 2018;273:13–20. https://doi.org/10.1016/j.jconrel.2018.01.015.
Kenniston JA, Faucette RR, Martik D, Comeau SR, Lindberg AP, Kopacz KJ, et al. Inhibition of plasma kallikrein by a highly specific active site blocking antibody. J Biol Chem. 2014;289(34):23596–608. https://doi.org/10.1074/jbc.M114.569061.
Chyung Y, Vince B, Iarrobino R, Sexton D, Kenniston J, Faucette R, et al. A phase 1 study investigating DX-2930 in healthy subjects. Ann Allergy Asthma Immunol. 2014;113(4):460–6.e2. https://doi.org/10.1016/j.anai.2014.05.028.
Nnane IP, Xu Z, Zhou H, Davis HM. Non-clinical pharmacokinetics, prediction of human pharmacokinetics and first-in-human dose selection for CNTO 5825, an anti-interleukin-13 monoclonal antibody. Basic Clin Pharmacol Toxicol. 2015;117(4):219–25. https://doi.org/10.1111/bcpt.12391.
van Hartingsveldt B, Nnane IP, Bouman-Thio E, Loza MJ, Piantone A, Davis HM, et al. Safety, tolerability and pharmacokinetics of a human anti-interleukin-13 monoclonal antibody (CNTO 5825) in an ascending single-dose first-in-human study. Br J Clin Pharmacol. 2013;75(5):1289–98. https://doi.org/10.1111/j.1365-2125.2012.04477.x.
Zia-Amirhosseini P, Minthorn E, Benincosa LJ, Hart TK, Hottenstein CS, Tobia LA, et al. Pharmacokinetics and pharmacodynamics of SB-240563, a humanized monoclonal antibody directed to human interleukin-5, in monkeys. J Pharmacol Exp Ther. 1999;291(3):1060–7.
Smith DA, Minthorn EA, Beerahee M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin-5 monoclonal antibody. Clin Pharmacokinet. 2011;50(4):215–27. https://doi.org/10.2165/11584340-000000000-00000.
PMDA. Adalimumab (Humira). 2008. https://www.pmda.go.jp/drugs/2008/P200800019/index.html. Accessed 1 Nov 2019.
PMDA. Omalizumab (Xolair). 2013. https://www.pmda.go.jp/drugs/2013/P201300105/index.html. Accessed 1 Nov 2019.
PMDA. Golimumab (Simponi). 2011. https://www.pmda.go.jp/drugs/2011/P201100114/index.html. Accessed 1 Nov 2019.
Zhuang Y, Lyn S, Lv Y, Xu Z, Bouman-Thio E, Masterson T, et al. Pharmacokinetics and safety of golimumab in healthy Chinese subjects following a single subcutaneous administration in a randomized phase I trial. Clin Drug Invest. 2013;33(11):795–800. https://doi.org/10.1007/s40261-013-0124-7.
PMDA. Secukinumab (Cosentyx). 2014. https://www.pmda.go.jp/drugs/2014/P201400171/index.html. Accessed 1 Nov 2019.
Lacy SE, Wu C, Ambrosi DJ, Hsieh CM, Bose S, Miller R, et al. Generation and characterization of ABT-981, a dual variable domain immunoglobulin (DVD-Ig(TM)) molecule that specifically and potently neutralizes both IL-1alpha and IL-1beta. mAbs. 2015;7(3):605–19. https://doi.org/10.1080/19420862.2015.1026501.
Kosloski MP, Goss S, Wang SX, Liu J, Loebbert R, Medema JK, et al. Pharmacokinetics and tolerability of a dual variable domain immunoglobulin ABT-981 against IL-1alpha and IL-1beta in healthy subjects and patients with osteoarthritis of the knee. J Clin Pharmacol. 2016;56(12):1582–90. https://doi.org/10.1002/jcph.764.
US FDA. Guselkumab (Tremfya). 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761061Orig1s000MultidisciplineR.pdf. Accessed 1 Nov 2019.
Zhuang Y, Calderon C, Marciniak SJ Jr, Bouman-Thio E, Szapary P, Yang TY, et al. First-in-human study to assess guselkumab (anti-IL-23 mAb) pharmacokinetics/safety in healthy subjects and patients with moderate-to-severe psoriasis. Eur J Clin Pharmacol. 2016;72(11):1303–10. https://doi.org/10.1007/s00228-016-2110-5.
PMDA. Ustekinumab (Stelara). 2011. https://www.pmda.go.jp/drugs/2011/P201100021/index.html. Accessed 1 Nov 2019.
Zhu Y, Wang Q, Frederick B, Bouman-Thio E, Marini JC, Keen M, et al. Comparison of the pharmacokinetics of subcutaneous ustekinumab between Chinese and non-Chinese healthy male subjects across two phase 1 studies. Clin Drug Invest. 2013;33(4):291–301. https://doi.org/10.1007/s40261-013-0072-2.
PMDA. Risankizumab (Skyrizi). 2019. https://www.pmda.go.jp/drugs/2019/P20190329001/112130000_23100AMX00299_A100_1.pdf. Accessed 1 Nov 2019.
Suleiman AA, Khatri A, Minocha M, Othman AA. Population pharmacokinetics of the interleukin-23 inhibitor risankizumab in subjects with psoriasis and Crohn's disease: analyses of phase I and II trials. Clin Pharmacokinet. 2019;58(3):375–87. https://doi.org/10.1007/s40262-018-0704-z.
Oyama S, Kitamura H, Kuramochi T, Higuchi Y, Matsushita H, Suzuki T, et al. Cynomolgus monkey model of interleukin-31-induced scratching depicts blockade of human interleukin-31 receptor A by a humanized monoclonal antibody. Exp Dermatol. 2018;27(1):14–211. https://doi.org/10.1111/exd.13236.
Nemoto O, Furue M, Nakagawa H, Shiramoto M, Hanada R, Matsuki S, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2016;174(2):296–304. https://doi.org/10.1111/bjd.14207.
Kock K, Pan WJ, Gow JM, Horner MJ, Gibbs JP, Colbert A, et al. Preclinical development of AMG 139, a human antibody specifically targeting IL-23. Br J Pharmacol. 2015;172(1):159–72. https://doi.org/10.1111/bph.12904.
Gibbs JP, Tsuji W, PAN WJ. Methods for treating psoriasis using an anti-IL-23 antibody. Patent (WO2014149425). 2014.
Muto A, Yoshihashi K, Takeda M, Kitazawa T, Soeda T, Igawa T, et al. Anti-factor IXa/X bispecific antibody (ACE910): hemostatic potency against ongoing bleeds in a hemophilia A model and the possibility of routine supplementation. J Thromb Haemost. 2014;12(2):206–13.
Uchida N, Sambe T, Yoneyama K, Fukazawa N, Kawanishi T, Kobayashi S, et al. A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood. 2016;127(13):1633–41. https://doi.org/10.1182/blood-2015-06-650226.
PMDA. Ixekizumab (Taltz). 2016. https://www.pmda.go.jp/drugs/2016/P20160725001/index.html. Accessed 1 Nov 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was fully supported by Chugai Pharmaceutical Co. Ltd.
Conflict of interest
Kenta Haraya and Tatsuhiko Tachibana are employees of Chugai Pharmaceutical Co. Ltd. and declare no potential conflicts of interest that might be relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All pharmacokinetic data is available in reference and all software is commercially available.
Code availability
Not applicable.
Authors’ contributions
K.H. and T.T. contributed to the study concept, data analysis and preparation of manuscript.
Rights and permissions
About this article
Cite this article
Haraya, K., Tachibana, T. Simple Approach to Accurately Predict Pharmacokinetics of Therapeutic Monoclonal Antibodies after Subcutaneous Injection in Humans. Clin Pharmacokinet 60, 111–120 (2021). https://doi.org/10.1007/s40262-020-00917-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00917-8