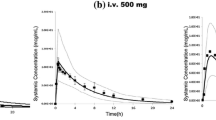
Abstract
Physiologically-based pharmacokinetic (PBPK) modelling provides an integrated framework to predict the disposition of small molecule drugs in children and is increasingly being used for dose recommendation and optimal design of paediatric studies and in regulatory submissions. Existing paediatric PBPK models can be adopted to describe the disposition of therapeutic proteins (TPs) in children by incorporating information on age-related changes of additional physiological and biological parameters (e.g. endogenous IgG, neonatal Fc receptor, lymph flow). In this study, physiological parameters were collated from literature and evaluated for any age-dependent trends. The age-dependent physiological parameters were used to construct a paediatric PBPK model for TPs. The model was then used to predict the pharmacokinetics of recombinant human erythropoietin (EPO), infliximab, etanercept, basiliximab, anakinra and enfuvirtide in paediatric subjects. The developed paediatric PBPK model predicted the drug concentration-time profiles reasonably well in full-term neonates (clinical PK data only available for EPO), infants, children and adolescents with the ratios of predicted over observed clearance values within 1.5-fold and 25 out of 26 clearance predictions were within 0.8- to 1.25-fold of the observed values. The clinically reported data are required to further assess the predictive accuracy of PK for Fc-containing proteins in term-born children younger than 2 months. This study demonstrates the ability of PBPK models accounting for age-dependent changes in relevant parameters to predict the pharmacokinetics of different types of TPs in paediatrics. The information gained from the PBPK models described here can facilitate our understanding of the complexities of TPs’ disposition during growth and development.








Similar content being viewed by others
Data Availability
The Simcyp Simulator is freely available, following completion of the relevant workshop, to approved members of academic institutions and other non-profit organisations for research and teaching purposes.
References
Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24:53–75.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931–56.
Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45:683–704.
Reichert JM. Marketed therapeutic antibodies compendium. MAbs. 2012;4:413–5.
Xu Z, Davis HM, Zhou H. Rational development and utilization of antibody-based therapeutic proteins in pediatrics. Pharmacol Ther. 2013;137:225–47.
Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78.
Rippe B, Haraldsson B. Fluid and protein fluxes across small and large pores in the microvasculature. Application of two-pore equations. Acta Physiol Scand. 1987;131:411–28.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25.
Brambell F, Hemmings W, Morris I. A theoretical model of γ-globulin catabolism. Nature. 1963;203:1352–5.
Wang W, Lu P, Fang Y, Hamuro L, Pittman T, Carr B, et al. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Disposition. 2011;39:1469–77.
Malik P, Edginton A. Pediatric physiology in relation to the pharmacokinetics of monoclonal antibodies. Expert Opin Drug Metab Toxicol. 2018;14:585–99.
Gill KL, Gardner I, Li L, Jamei M. A bottom-up whole-body physiologically based pharmacokinetic model to mechanistically predict the tissue distribution and the rate of subcutaneous absorption of therapeutic proteins. AAPS J. 2015;18:156–70.
Li L, Gardner I, Dostalek M, Jamei M. Simulation of monoclonal antibody pharmacokinetics in humans using a minimal physiologically based model. AAPS J. 2014;16:1097–109.
Ohlson M, Sorensson J, Haraldsson B. A gel-membrane model of glomerular charge and size selectivity in series. Am J Physiol Renal Physiol. 2001;280:F396–405.
Deen WM, Bridges CR, Brenner BM, Myers BD. Heteroporous model of glomerular size selectivity: application to normal and nephrotic humans. Am J Phys. 1985;249:F374–89.
Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45:490–7.
Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Food and Drug Administration. BLA99-1490 (anakinra, Kineret™) clinical pharmacology review. 2000. Available from: www.accessdata.fda.gov/drugsatfda_docs/nda/2001/103950-0_Kineret_Biopharmr.PDF.
Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol. 2010;184:1968–76.
Sawada K, Krantz SB, Sawyer ST, Civin CI. Quantitation of specific binding of erythropoietin to human erythroid colony-forming cells. J Cell Physiol. 1988;137:337–45.
Kaymakcalan Z, Sakorafas P, Bose S, Scesney S, Xiong L, Hanzatian DK, et al. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol. 2009;131:308–16.
Gross AW, Lodish HF. Cellular trafficking and degradation of erythropoietin and novel erythropoiesis stimulating protein (NESP). J Biol Chem. 2006;281:2024–32.
Stepensky D. Local versus systemic anti-tumour necrosis factor-alpha effects of adalimumab in rheumatoid arthritis: pharmacokinetic modelling analysis of interaction between a soluble target and a drug. Clin Pharmacokinet. 2012;51:443–55.
Meibohm B, Zhou H. Characterizing the impact of renal impairment on the clinical pharmacology of biologics. J Clin Pharmacol. 2012;52:54S–62S.
Mager DE, Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res. 2005;22:1589–96.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28:507–32.
Krzyzanski W, Wyska E. Pharmacokinetics and pharmacodynamics of erythropoietin receptor in healthy volunteers. Naunyn Schmiedeberg’s Arch Pharmacol. 2008;377:637–45.
Fisher J, Nakashima J. The role of hypoxia in renal production of erythropoietin. Cancer. 1992;70:928–39.
Aksu G, Genel F, Koturoglu G, Kurugol Z, Kutukculer N. Serum immunoglobulin (IgG, IgM, IgA) and IgG subclass concentrations in healthy children: a study using nephelometric technique. Turk J Pediatr. 2006;48:19–24.
Allansmith M, McClellan B, Butterworth M, Maloney J. The development of immunoglobulinlevels in man. J Pediatr. 1968;72:276–90.
Isaacs D, Altman D, Tidmarsh C, Valman H, Webster A. Serum immunoglobulin concentrations in preschool children measured by laser nephelometry: reference ranges for IgG, IgA. IgM J Clin Pathol. 1983;36:1193–6.
Jazayeri MH, Pourfathollah AA, Rasaee MJ, Porpak Z, Jafari ME. The concentration of total serum IgG and IgM in sera of healthy individuals varies at different age intervals. Biomedicine & Aging Pathology. 2013;3:241–5.
Jolliff C, Cost K, Stivrins P, Grossman PP, Nolte CR, Franco S, et al. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin Chem. 1982;28:126–8.
Kardar G, Oraei M, Shahsavani M, Namdar Z, Kazemisefat G, Ashtiani MH, et al. Reference intervals for serum immunoglobulins IgG, IgA, IgM and complements C3 and C4 in Iranian healthy children. Iran J Public Health. 2012;41:59–63.
Lau Y, Jones B, Ng K, Yeung C. Percentile ranges for serum IgG subclass concentrations in healthy Chinese children. Clin Exp Immunol. 1993;91:337–41.
Lepage N, Huang S-HS, Nieuwenhuys E, Filler G. Pediatric reference intervals for immunoglobulin G and its subclasses with Siemens immunonephelometric assays. Clin Biochem. 2010;43:694–6.
Malvy D, Poveda J, Debruyne M, Montagnon B, Burtschy B, Herbert C, et al. Laser immunonephelometry reference intervals for eight serum proteins in healthy children. Clin Chem. 1992;38:394–9.
Sitcharungsi R, Ananworanich J, Vilaiyuk S, Apornpong T, Bunupuradah T, Pornvoranunt A, Nouanthong P, Phasomsap C, Khupulsup K, Pancharoen C, Puthanakit T, Shearer WT, Benjaponpitak S, on behalf of the HIV-NAT 108 Study Group Nephelometry determined serum immunoglobulin isotypes in healthy Thai children aged 2–15 years. Microbiol Immunol 2012;56:117–122.
Waldmann TA, Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110.
Antohe F, Rădulescu LA, Gafencu A, Ghetie V, Simionescu M. Expression of functionally active FcRn and the differentiated bidirectional transport of IgG in human placental endothelial cells. Hum Immunol. 2001;62:93–105.
Morell A, Skvaril F, Wu H, Barandun S. IgG subclasses: development of the serum concentrations in “normal” infants and children. J Pediatr. 1972;80:960–4.
Barr M, Glenny AT, Randall KJ. Concentration of diphtheria antitoxin in cord blood and rate of loss in babies. Lancet. 1949;2:324–6.
Birke G, Liljedahl SO, Olhagen B, Plantin LO, Ahlinder S. Catabolism and distribution of gamma-globulin. A preliminary study with 131 I-labelled gammaglobulin. Acta Med Scand. 1963;173:589–603.
Black FL, Berman LL, Borgono JM, Capper RA, Carvalho AA, Collins C, et al. Geographic variation in infant loss of maternal measles antibody and in prevalence of rubella antibody. Am J Epidemiol. 1986;124:442–52.
Da Silva MM, Prem KA, Johnson EA, McKelvey JL, Syverton JT. Response of pregnant women and their infants to poliomyelitis vaccine: distribution of poliovirus antibody in pregnant women before and after vaccination; transfer, persistence, and induction of antibodies in infants. J Am Med Assoc. 1958;168:1–5.
Neil JM, Gaspari EL, Richardson LV, Sugg JY. Diphtheria antibodies transmitted from mother to child. J Immunol. 1932;22:117–24.
Noya FJ, Rench MA, Garcia-Prats JA, Jones TM, Baker CJ. Disposition of an immunoglobulin intravenous preparation in very low birth weight neonates. J Pediatr. 1988;112:278–83.
Sarvas H, Seppälä I, Kurikka S, Siegberg R, Mäkelä O. Half-life of the maternal IgG1 allotype in infants. J Clin Immunol. 1993;13:145–51.
Sato H, Albrecht P, Reynolds DW, Stagno S, Ennis FA. Transfer of measles, mumps, and rubella antibodies from mother to infant. Its effect on measles, mumps, and rubella immunization. Am J Dis Child. 1979;133:1240–3.
Solomon A, Waldmann TA, Fahey JL. Clinical and experimental metabolism of normal 6.6s gamma-globulin in normal subjects and in patients with macroglobulinemia and multiple myeloma. J Lab Clin Med. 1963;62:1–17.
Stiehm ER, Vaerman JP, Fudenberg HH. Plasma infusions in immunologic deficiency states: metabolic and therapeutic studies. Blood. 1966;28:918–37.
Strean GJ, Gelfand MM, Pavilanis V, Sternberg J. Maternal-fetal relationships: placental transmission of poliomyelitis antibodies in newborn. Can Med Assoc J. 1957;77:315–23.
Vaisberg A, Alvarez JO, Hernandez H, Guillen D, Chu P, Colarossi A. Loss of maternally acquired measles antibodies in well-nourished infants and response to measles vaccination. Peru Am J Public Health. 1990;80:736–8.
Waldmann TA, Schwab PJ. Igg (7 S gamma globulin) metabolism in hypogammaglobulinemia: studies in patients with defective gamma globulin synthesis, gastrointestinal protein loss, or both. J Clin Invest. 1965;44:1523–33.
Weisman LE, Fischer GW, Hemming VG, Peck CC. Pharmacokinetics of intravenous immunoglobulin (sandoglobulin) in neonates. Pediatr Infect Dis J. 1986;5:S185–S8.
Wiener AS. The half-life of passively acquired antibody globulin molecules in infants. J Exp Med. 1951;94:213–21.
Fan Y-Y, Avery LB, Wang M, O’Hara DM, Leung S, Neubert H. Tissue expression profile of human neonatal Fc receptor (FcRn) in Tg32 transgenic mice. MAbs. 2016;8:848–53.
Israel E, Taylor S, Wu Z, Mizoguchi E, Blumberg R, Bhan A, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74.
Cianga C, Cianga P, Plamadeala P, Amalinei C. Nonclassical major histocompatibility complex I-like Fc neonatal receptor (FcRn) expression in neonatal human tissues. Hum Immunol. 2011;72:1176–87.
Luscieti P, Hubschmid T, Cottier H, Hess M, Sobin L. Human lymph node morphology as a function of age and site. J Clin Pathol. 1980;33:454–61.
Vierordt H. Anatomische, physiologische und physikalische Daten und Tabellen zum Gebrauche für Mediziner: Jena: G: Fischer; 1888.
Johnson S, Vander Straten M, Parellada J, Schnakenberg W, Gest A. Thoracic duct function in fetal, newborn, and adult sheep. Lymphology. 1996;29:50–6.
Taylor PM, Boonyaprakob U, Waterman V, Watson D, Lopata E. Clearances of plasma proteins from pulmonary vascular beds of adult dogs and pups. Am J Phys. 1967;213:441–9.
Braun A, Ding R, Seidel C, Fies T, Kurtz A, Schärer K. Pharmacokinetics of recombinant human erythropoietin applied subcutaneously to children with chronic renal failure. Pediatr Nephrol. 1993;7:61–4.
Kling PJ, Widness JA, Guillery EN, Veng-Pedersen P, Peters C, DeAlarcon PA. Pharmacokinetics and pharmacodynamics of erythropoietin during therapy in an infant with renal failure. J Pediatr. 1992;121:822–5.
Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130:683–91.
Adedokun OJ, Xu Z, Padgett L, Blank M, Johanns J, Griffiths A, et al. Pharmacokinetics of infliximab in children with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2013;19:2753–62.
Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, et al. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn’s disease. Am J Gastroenterol. 2003;98:833–8.
Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, et al. Infliximab treatment of intravenous immunoglobulin–resistant Kawasaki disease. J Pediatr. 2008;153:833–8.
Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096–106.
Li CR, Yang XQ, Shen J, Li YB, Jiang LP. Immunoglobulin G subclasses in serum and circulating immune complexes in patients with Kawasaki syndrome. Pediatr Infect Dis J. 1990;9:544–7.
Niu L, An XJ, Fu MY, He XH, Wang QW. Observation of Kawasaki disease-related indexes and the study of relationship between myocardial enzyme changes and coronary artery lesions. Eur Rev Med Pharmacol Sci. 2015;19:4407–10.
Choueiter NF, Olson AK, Shen DD, Portman MA. Prospective open-label trial of etanercept as adjunctive therapy for Kawasaki disease. J Pediatr. 2010;157:960–6.e1.
Yim D-S, Zhou H, Buckwalter M, Nestorov I, Peck CC, Lee H. Population pharmacokinetic analysis and simulation of the time-concentration profile of etanercept in pediatric patients with juvenile rheumatoid arthritis. J Clin Pharmacol. 2005;45:246–56.
Zhou SY, Shu C, Korth-Bradley J, Raible D, Palmisano M, Wadjula J, et al. Integrated population pharmacokinetics of etanercept in healthy subjects and in patients with rheumatoid arthritis ankylosing spondylitis. J Clin Pharmacol. 2011;51:864–75.
Kovarik JM, Offner G, Broyer M, Niaudet P, Loirat C, Mentser M, et al. A rational dosing algorithm for basiliximab (Simulect) in pediatric renal transplantation based on pharmacokinetic-dynamic evaluations. Transplantation. 2002;74:966–71.
Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Food and Drug Administration. BLA97-1251 (basiliximab, Simulect™) clinical pharmacology Review. 1998. Available from: www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm113364.pdf.
Urien S, Bardin C, Bader-Meunier B, Mouy R, Compeyrot-Lacassagne S, Foissac F, et al. Anakinra pharmacokinetics in children and adolescents with systemic-onset juvenile idiopathic arthritis and autoinflammatory syndromes. BMC Pharmacol Toxicol. 2013;14:40.
Ilowite N, Porras O, Reiff A, Rudge S, Punaro M, Martin A, et al. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol. 2008;28:129–37.
Soy D, Aweeka FT, Church JA, Cunningham CK, Palumbo P, Kosel BW, et al. Population pharmacokinetics of enfuvirtide in pediatric patients with human immunodeficiency virus: searching for exposure-response relationships. Clin Pharmacol Ther. 2003;74:569–80.
Bellibas SE, Siddique Z, Dorr A, Bertasso A, Sista P, Kolis SJ, et al. Pharmacokinetics of enfuvirtide in pediatric human immunodeficiency virus 1-infected patients receiving combination therapy. Pediatr Infect Dis J. 2004;23:1137–41.
Björkman S. Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol. 2005;59:691–704.
Johnson TN, Rostami-Hodjegan A. Resurgence in the use of physiologically based pharmacokinetic models in pediatric clinical pharmacology: parallel shift in incorporating the knowledge of biological elements and increased applicability to drug development and clinical practice. Pediatr Anesth. 2011;21:291–301.
Bouzom F, Walther B. Pharmacokinetic predictions in children by using the physiologically based pharmacokinetic modelling. Fundam Clin Pharmacol. 2008;22:579–87.
Cao Y, Balthasar JP, Jusko WJ. Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2013;40:597–607.
Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39:67–86.
Bellini C, Boccardo F, Campisi C, Villa G, Taddei G, Traggiai C, et al. Lymphatic dysplasias in newborns and children: the role of lymphoscintigraphy. J Pediatr. 2008;152:587–9.
Bellini C, Villa G, Sambuceti G, Traggiai C, Campisi C, Bellini T, et al. Lymphoscintigraphy patterns in newborns and children with congenital lymphatic dysplasia. Lymphology. 2014;47:28–39.
Hardiansyah D, Ng CM. Effects of the FcRn developmental pharmacology on the pharmacokinetics of therapeutic monoclonal IgG antibody in pediatric subjects using minimal physiologically-based pharmacokinetic modelling. MAbs. 2018;10:1144–56.
Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179:4580–8.
Chen X, DuBois DC, Almon RR, Jusko WJ. Biodistribution of etanercept to tissues and sites of inflammation in arthritic rats. Drug Metab Dispos. 2015;43:898–907.
Gómez-Mantilla JD, Trocóniz IF, Parra-Guillén Z, Garrido MJ. Review on modeling anti-antibody responses to monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2014;41:523–36.
Gorovits B, Baltrukonis DJ, Bhattacharya I, Birchler MA, Finco D, Sikkema D, et al. Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab. Clin Exp Immunol. 2018;192:348–65.
Strik AS, van den Brink GR, Ponsioen C, Mathot R, Lowenberg M, D’Haens GR. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:1128–34.
Mahmood I. Interspecies scaling of protein drugs: prediction of clearance from animals to humans. J Pharm Sci. 2004;93:177–85.
Tang H, Mayersohn M. A global examination of allometric scaling for predicting human drug clearance and the prediction of large vertical allometry. J Pharm Sci. 2006;95:1783–99.
Hanke N, Kunz C, Thiemann M, Fricke H, Lehr T. Translational PBPK modeling of the protein therapeutic and CD95L inhibitor asunercept to develop dose recommendations for its first use in pediatric glioblastoma patients. Pharmaceutics. 2019;11:E152.
Malik PRV, Edginton AN. Physiologically-based pharmacokinetic modelling versus allometric scaling for the prediction of infliximab pharmacokinetics in pediatric patients. CPT Pharmacometrics Syst Pharmacol. 2019;8:835–44.
Acknowledgments
The authors thank Eleanor Savill for her assistance in the preparation and submission of the article and Dr. Mian Zhang for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Felix Stader has no conflict of interest to declare. Xian Pan, Khaled Abduljalil, Katherine L. Gill, Trevor N. Johnson, Iain Gardner and Masoud Jamei are employees of Certara UK Limited, Simcyp Division.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 910 kb)
Rights and permissions
About this article
Cite this article
Pan, X., Stader, F., Abduljalil, K. et al. Development and Application of a Physiologically-Based Pharmacokinetic Model to Predict the Pharmacokinetics of Therapeutic Proteins from Full-term Neonates to Adolescents. AAPS J 22, 76 (2020). https://doi.org/10.1208/s12248-020-00460-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00460-1