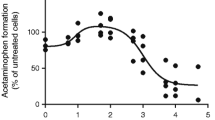
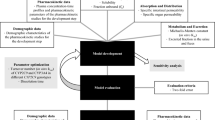
Abstract
A physiologically based pharmacokinetic (PBPK) model was used to simulate the impact of elevated levels of interleukin (IL)-6 on the exposure of several orally administered cytochrome P450 (CYP) probe substrates (caffeine, S-warfarin, omeprazole, dextromethorphan, midazolam, and simvastatin). The changes in exposure of these substrates in subjects with rheumatoid arthritis (and hence elevated IL-6 levels) compared with healthy subjects were predicted with a reasonable degree of accuracy. The PBPK model was then used to simulate the change in oral exposure of the probe substrates in North European Caucasian, Chinese, and Japanese population of patients with neuromyelitis optica (NMO) or NMO spectrum disorder with elevated plasma IL-6 levels (up to 100 pg/mL). Moderate interactions [mean AUC fold change, ≤ 2.08 (midazolam) or 2.36 (simvastatin)] was predicted for CYP3A4 probe substrates and weak interactions (mean AUC fold change, ≤ 1.29–1.97) were predicted for CYP2C19, CYP2C9, and CYP2D6 substrates. No notable interaction was predicted with CYP1A2. Although ethnic differences led to differences in simulated exposure for some of the probe substrates, there were no marked differences in the predicted magnitude of the change in exposure following IL-6–mediated suppression of CYPs. Decreased levels of serum albumin (as reported in NMO patients) had little impact on the magnitude of the simulated IL-6–mediated drug interactions. This PBPK modeling approach allowed us to leverage knowledge from different disease and ethnic populations to make predictions of cytokine-related DDIs in a rare disease population where actual clinical studies would otherwise be difficult to conduct.








Similar content being viewed by others
References
Kempf L, Goldsmith JC, Temple R. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A. 2018;176:773–83.
Administration USFaD. US FDA, 21 CFR Part 316, Designating an orphan product: drugs and biological products.
Agency EM. European Union EC Regulation No 141/2000; Orphan designation.
Frye RF, Schneider VM, Frye CS, Feldman AM. Plasma levels of TNF-alpha and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J Card Fail. 2002;8:315–9.
Sunman JA, Hawke RL, LeCluyse EL, Kashuba AD. Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab Dispos. 2004;32:359–63.
Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br J Cancer. 2002;87:277–80.
Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease–drug–drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89:735–40.
Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–22.
Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–93.
Wang CWS, Khan M, Mao-Draayer Y. Interleukin-6 receptor: a novel therapeutic target for neuromyelitis optica. Brain Disord Ther. 2015;4:e119.
Uzawa A, Mori M, Arai K, Sato Y, Hayakawa S, Masuda S, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16:1443–52.
Araki M, Aranami T, Matsuoka T, Nakamura M, Miyake S, Yamamura T. Clinical improvement in a patient with neuromyelitis optica following therapy with the anti-IL-6 receptor monoclonal antibody tocilizumab. Mod Rheumatol. 2013;23:827–31.
Ringelstein M, Ayzenberg I, Harmel J, Lauenstein AS, Lensch E, Stogbauer F, et al. Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurol. 2015;72:756–63.
Morgan ET. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther. 2009;85:434–8.
Zhang XSC, Grange S, Terao K, Miya K, Kivitz A, Marino M. Disease–drug interaction studies of tocilizumab with cytochrome P450 substrates in vitro and in vivo. Clin Pharmacol Ther. 2009;85:S59.
Zhuang Y, de Vries DE, Xu Z, Marciniak SJ Jr, Chen D, Leon F, et al. Evaluation of disease-mediated therapeutic protein–drug interactions between an anti-interleukin-6 monoclonal antibody (sirukumab) and cytochrome P450 activities in a phase 1 study in patients with rheumatoid arthritis using a cocktail approach. J Clin Pharmacol. 2015;55:1386–94.
Dickmann LJ, Patel SK, Wienkers LC, Slatter JG. Effects of interleukin 1beta (IL-1beta) and IL-1beta/interleukin 6 (IL-6) combinations on drug metabolizing enzymes in human hepatocyte culture. Curr Drug Metab. 2012;13:930–7.
Jiang X, Zhuang Y, Xu Z, Wang W, Zhou H. Development of a physiologically based pharmacokinetic model to predict disease-mediated therapeutic protein–drug interactions: modulation of multiple cytochrome P450 enzymes by interleukin-6. AAPS J. 2016;18:767–76.
Machavaram KK, Almond LM, Rostami-Hodjegan A, Gardner I, Jamei M, Tay S, et al. A physiologically based pharmacokinetic modeling approach to predict disease–drug interactions: suppression of CYP3A by IL-6. Clin Pharmacol Ther. 2013;94:260–8.
Xu Y, Hijazi Y, Wolf A, Wu B, Sun YN, Zhu M. Physiologically based pharmacokinetic model to assess the influence of blinatumomab-mediated cytokine elevations on cytochrome P450 enzyme activity. CPT Pharmacometrics Syst Pharmacol. 2015;4:507–15.
Barter ZE, Tucker GT, Rowland-Yeo K. Differences in cytochrome p450-mediated pharmacokinetics between Chinese and Caucasian populations predicted by mechanistic physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2013;52:1085–100.
Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871–7.
Kim K, Johnson JA, Derendorf H. Differences in drug pharmacokinetics between east Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol. 2004;44:1083–105.
Jarius S, Paul F, Franciotta D, Ruprecht K, Ringelstein M, Bergamaschi R, et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J Neurol Sci. 2011;306:82–90.
Peng F, Yang Y, Liu J, Jiang Y, Zhu C, Deng X, et al. Low antioxidant status of serum uric acid, bilirubin and albumin in patients with neuromyelitis optica. Eur J Neurol. 2012;19:277–83.
de Jong J, Skee D, Hellemans P, Jiao J, de Vries R, Swerts D, et al. Single-dose pharmacokinetics of ibrutinib in subjects with varying degrees of hepatic impairment. Leuk Lymphoma. 2017;58:185–94.
Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Asp Med. 2012;33:209–90.
Rasool MF, Khalil F, Laer S. Optimizing the clinical use of carvedilol in liver cirrhosis using a physiologically based pharmacokinetic modeling approach. Eur J Drug Metab Pharmacokinet. 2017;42:383–96.
Barros PO, Cassano T, Hygino J, Ferreira TB, Centuriao N, Kasahara TM, et al. Prediction of disease severity in neuromyelitis optica by the levels of interleukin (IL)-6 produced during remission phase. Clin Exp Immunol. 2016;183:480–9.
InvivoGen. Recombinant human IL-6 2018. Available from: https://www.invivogen.com/human-il6. Accessed 10 Dec 2018.
Rowland Yeo K, Jamei M, Yang J, Tucker GT, Rostami-Hodjegan A. Physiologically based mechanistic modelling to predict complex drug–drug interactions involving simultaneous competitive and time-dependent enzyme inhibition by parent compound and its metabolite in both liver and gut—the effect of diltiazem on the time-course of exposure to triazolam. Eur J Pharm Sci. 2010;39:298–309.
Howgate EM, Rowland Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data. I: impact of inter-individual variability. Xenobiotica. 2006;36:473–97.
Jacob A, Panicker J, Lythgoe D, Elsone L, Mutch K, Wilson M, et al. The epidemiology of neuromyelitis optica amongst adults in the Merseyside county of United Kingdom. J Neurol. 2013;260:2134–7.
Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012;69:1176–80.
Pandit L, Asgari N, Apiwattanakul M, Palace J, Paul F, Leite MI, et al. Demographic and clinical features of neuromyelitis optica: a review. Mult Scler. 2015;21:845–53.
Papais-Alvarenga RM, Miranda-Santos CM, Puccioni-Sohler M, de Almeida AM, Oliveira S, Basilio De Oliveira CA, et al. Optic neuromyelitis syndrome in Brazilian patients. J Neurol Neurosurg Psychiatry. 2002;73:429–35.
Uchida T, Mori M, Uzawa A, Masuda H, Muto M, Ohtani R, et al. Increased cerebrospinal fluid metalloproteinase-2 and interleukin-6 are associated with albumin quotient in neuromyelitis optica: their possible role on blood–brain barrier disruption. Mult Scler. 2017;23:1072–84.
Wang Y, Wu A, Chen X, Zhang L, Lin Y, Sun S, et al. Comparison of clinical characteristics between neuromyelitis optica spectrum disorders with and without spinal cord atrophy. BMC Neurol. 2014;14:246.
Wang Y, Zhou Y, Sun X, Lu T, Wei L, Fang L, et al. Cytokine and chemokine profiles in patients with neuromyelitis optica spectrum disorder. Neuroimmunomodulation. 2016;23:352–8.
Bertilsson L, Tybring G, Widen J, Chang M, Tomson T. Carbamazepine treatment induces the CYP3A4 catalysed sulphoxidation of omeprazole, but has no or less effect on hydroxylation via CYP2C19. Br J Clin Pharmacol. 1997;44:186–9.
Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA. The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans. Clin Pharmacol Ther. 1996;60:295–307.
Chung E, Nafziger AN, Kazierad DJ, Bertino JS Jr. Comparison of midazolam and simvastatin as cytochrome P450 3A probes. Clin Pharmacol Ther. 2006;79:350–61.
Cysneiros RM, Farkas D, Harmatz JS, von Moltke LL, Greenblatt DJ. Pharmacokinetic and pharmacodynamic interactions between zolpidem and caffeine. Clin Pharmacol Ther. 2007;82:54–62.
Hassan-Alin M, Andersson T, Niazi M, Rohss K. A pharmacokinetic study comparing single and repeated oral doses of 20 mg and 40 mg omeprazole and its two optical isomers, S-omeprazole (esomeprazole) and R-omeprazole, in healthy subjects. Eur J Clin Pharmacol. 2005;60:779–84.
Krishna G, Ma L, Prasad P, Moton A, Martinho M, O'Mara E. Effect of posaconazole on the pharmacokinetics of simvastatin and midazolam in healthy volunteers. Expert Opin Drug Metab Toxicol. 2012;8:1–10.
Krishna R, Stypinski D, Ali M, Garg A, Cote J, Maes A, et al. Lack of a meaningful effect of anacetrapib on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2012;74:116–24.
Kyrklund C, Backman JT, Kivisto KT, Neuvonen M, Laitila J, Neuvonen PJ. Rifampin greatly reduces plasma simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 2000;68:592–7.
Liao S, Palmer M, Fowler C, Nayak RK. Absence of an effect of levofloxacin on warfarin pharmacokinetics and anticoagulation in male volunteers. J Clin Pharmacol. 1996;36:1072–7.
Randinitis EJ, Alvey CW, Koup JR, Rausch G, Abel R, Bron NJ, et al. Drug interactions with clinafloxacin. Antimicrob Agents Chemother. 2001;45:2543–52.
Stoch SA, Friedman E, Maes A, Yee K, Xu Y, Larson P, et al. Effect of different durations of ketoconazole dosing on the single-dose pharmacokinetics of midazolam: shortening the paradigm. J Clin Pharmacol. 2009;49:398–406.
Templeton I, Peng CC, Thummel KE, Davis C, Kunze KL, Isoherranen N. Accurate prediction of dose-dependent CYP3A4 inhibition by itraconazole and its metabolites from in vitro inhibition data. Clin Pharmacol Ther. 2010;88:499–505.
Yu RZ, Geary RS, Flaim JD, Riley GC, Tribble DL, van Vliet AA, et al. Lack of pharmacokinetic interaction of mipomersen sodium (ISIS 301012), a 2'-O-methoxyethyl modified antisense oligonucleotide targeting apolipoprotein B-100 messenger RNA, with simvastatin and ezetimibe. Clin Pharmacokinet. 2009;48:39–50.
Abdul Manap R, Wright CE, Gregory A, Rostami-Hodjegan A, Meller ST, Kelm GR, et al. The antitussive effect of dextromethorphan in relation to CYP2D6 activity. Br J Clin Pharmacol. 1999;48:382–7.
Terao KTT, Suzaki M, Ishida Y, Amamoto T, Amamoto H, Higuchi S, et al. Drug–disease interaction study of tocilizumab in patients with rheumatoid arthritis—IL-6 signal inhibition normalised cytochrome P-450 enzymes expression which was reduced by inflammation. Int J Rheum Dis. 2010;13:95–105.
Febvre-James M, Bruyere A, Le Vee M, Fardel O. The JAK1/2 inhibitor ruxolitinib reverses interleukin-6-mediated suppression of drug-detoxifying proteins in cultured human hepatocytes. Drug Metab Dispos. 2018;46:131–40.
Migita K, Izumi Y, Jiuchi Y, Kozuru H, Kawahara C, Izumi M, et al. Effects of Janus kinase inhibitor tofacitinib on circulating serum amyloid a and interleukin-6 during treatment for rheumatoid arthritis. Clin Exp Immunol. 2014;175:208–14.
Tabarroki A, Lindner DJ, Visconte V, Zhang L, Rogers HJ, Parker Y, et al. Ruxolitinib leads to improvement of pulmonary hypertension in patients with myelofibrosis. Leukemia. 2014;28:1486–93.
Gorski JC, Hall SD, Becker P, Affrime MB, Cutler DL, Haehner-Daniels B. In vivo effects of interleukin-10 on human cytochrome P450 activity. Clin Pharmacol Ther. 2000;67:32–43.
Huhn RD, Radwanski E, Gallo J, Affrime MB, Sabo R, Gonyo G, et al. Pharmacodynamics of subcutaneous recombinant human interleukin-10 in healthy volunteers. Clin Pharmacol Ther. 1997;62:171–80.
Lee EB, Daskalakis N, Xu C, Paccaly A, Miller B, Fleischmann R, et al. Disease–drug interaction of sarilumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacokinet. 2017;56:607–15.
Acknowledgments
The authors thank Certara’s library team for their assistance in the preparation and submission of this paper. The authors thank Hajime Ito for his assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
K.K.M., C.E.-T., K.T., K.L.G., O.J.H., I.G., N.P., and P.S.D. wrote the manuscript.
K.K.M., C.E.-T., K.L.G., I.G., N.P., and P.S.D. designed the research.
K.K.M., K.L.G., O.J.H., and I.G. performed the research.
K.K.M., K.L.G., and I.G. analyzed the research.
Corresponding author
Ethics declarations
Conflict of Interest
K.K.M., K.L.G., and I.G. are employees of Certara UK Limited, Simcyp Division, Sheffield, United Kingdom.
C.E.-T. and K.T. are employees of Chugai Pharmaceutical Co., Ltd., Tokyo, Japan.
N.P. and P.S.D. are employees of F. Hoffmann–La Roche Ltd., Basel, Switzerland.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 716 KB)
Rights and permissions
About this article
Cite this article
Machavaram, K.K., Endo-Tsukude, C., Terao, K. et al. Simulating the Impact of Elevated Levels of Interleukin-6 on the Pharmacokinetics of Various CYP450 Substrates in Patients with Neuromyelitis Optica or Neuromyelitis Optica Spectrum Disorders in Different Ethnic Populations. AAPS J 21, 42 (2019). https://doi.org/10.1208/s12248-019-0309-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0309-y