Abstract
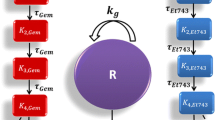
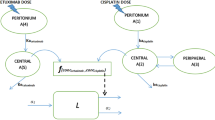
The pharmacodynamic interactions among trifluoperazine (TFP), gemcitabine (GEM), and paclitaxel (PTX) were assessed in pancreatic cancer cells (PANC-1). The phenothiazine TFP was chosen for its potential activity on cancer stem cells, while GEM and PTX cause apoptosis. Effects of each drug alone and in various combinations on cell growth inhibition of PANC-1 cells were studied in vitro to determine the drug-specific parameters and assess the nature of drug interactions. Joint inhibition (JI) and competitive inhibition (CI) equations were applied with a ψ interaction term. TFP fully inhibited growth of cells (Imax = 1) with an IC50 = 9887 nM. Near-maximum inhibition was achieved for GEM (Imax = 0.825) and PTX (Imax= 0.844) with an IC50 = 17.4 nM for GEM and IC50 = 7.08 nM for PTX. Estimates of an interaction term ψ revealed that the combination of TFP-GEM was apparently synergistic; close to additivity, the combination TFP-PTX was antagonistic. The interaction of GEM-PTX was additive, and TFP-GEM-PTX was synergistic but close to additive. The combination of TFP IC60–GEM IC60–PTX IC60 seemed optimal in producing inhibition of PANC-1 cells with an inhibitory effect of 82.1–90.2%. The addition of ψ terms to traditional interaction equations allows assessment of the degree of perturbation of assumed mechanisms.






Similar content being viewed by others
References
Cancer.net. Pancreatic cancer: treatment options: ASCO (American Society of Clinical Oncology); [updated 12/2016; cited 2017 6/6]. Available from: http://www.cancer.net/cancer-types/pancreatic-cancer/treatment-options.
ANSM. Summary of product characteristics (Gemcitabine): Agence nationale de sécurité du médicament et des produits de santé; [updated 29/11/2013; cited 2017 16 june]. Available from: http://agence-prd.ansm.sante.fr/php/ecodex/rcp/R0233325.htm.
Cancer.gov. Combination of Nab-paclitaxel and gemcitabine improves survival in patients with metastatic pancreatic cancer: National Cancer Institute; [updated 15/11/13; cited 2017 07/06]. Available from: https://www.cancer.gov/types/pancreatic/research/nab-paclitaxel-gemcitabine.
EMA. Summary of product characteristics (Nab-paclitaxel): European Medicine Agency; [cited 2017 16 june]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000778/WC500020435.pdf.
Oliveras-Ferraros C, Vazquez-Martin A, Colomer R, De Llorens R, Brunet J, Menendez JA. Sequence-dependent synergism and antagonism between paclitaxel and gemcitabine in breast cancer cells: the importance of scheduling. Int J Oncol. 2008;32(1):113–20.
Shu CH, Yang WK, Shih YL, Kuo ML, Huang TS. Cell cycle G2/M arrest and activation of cyclin-dependent kinases associated with low-dose paclitaxel-induced sub-G1 apoptosis. Apoptosis. 1997;2(5):463–70.
Polischouk AG, Holgersson A, Zong D, Stenerlöw B, Karlsson HL, Möller L, et al. The antipsychotic drug trifluoperazine inhibits DNA repair and sensitizes non-small cell lung carcinoma cells to DNA double-strand break–induced cell death. Mol Cancer Ther. 2007;6(8):2303–9.
Yuan K, Yong S, Xu F, Zhou T, McDonald JM, Chen Y. Calmodulin antagonists promote TRA-8 therapy of resistant pancreatic cancer. Oncotarget. 2015;6(28):25308–19.
Yeh C-T, Wu ATH, Chang PMH, Chen K-Y, Yang C-N, Yang S-C, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med. 2012;186(11):1180–8.
Cheng H, Lin C-F, Shih J-H, Wu ACH, inventors; Acenda Pharma, Inc, assignee. Phenothiazine derivatives and methods of use thereof. United States patent US 9695138B1. 2017/07/04.
Koch G, Schropp J, Jusko WJ. Assessment of non-linear combination effect terms for drug-drug interactions. J Pharmacokinet Pharmacodyn. 2016;43(5):461–79.
Ariens EJ, Van Rossum JM, Simonis AM. Affinity, intrinsic activity and drug interactions. Pharmacol Rev. 1957;9(2):218–36.
Earp J, Krzyzanski W, Chakraborty A, Zamacona MK, Jusko WJ. Assessment of drug interactions relevant to pharmacodynamic indirect response models. J Pharmacokinet Pharmacodyn. 2004;31(5):345–80.
Chakraborty A, Jusko WJ. Pharmacodynamic interaction of recombinant human interleukin-10 and prednisolone using in vitro whole blood lymphocyte proliferation. J Pharm Sci. 2002;91(5):1334–42.
D'Argenio DZ, Schumitzky A, Wang X. ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource; 2009.
Zhao L, Au JLS, Wientjes MG. Comparison of methods for evaluating drug-drug interaction. Front Biosci Elite Ed. 2010;2:241–9.
Yao, J, Cai H-h, Wei J-s, An Y, Ji Z-l, Lu Z-p, et al. Side population in the pancreatic cancer cell lines SW1990 and CFPAC-1 is enriched with cancer stem-like cells. Oncol Rep. 2010;23(5):1375–82.
Hait WN, Pierson NR. Comparison of the efficacy of a phenothiazine and a bisquinaldinium calmodulin antagonist against multidrug-resistant P388 cell lines. Cancer Res. 1990;50(4):1165–9.
Midha KK, Korchinski ED, Verbeeck RK, Roscoe RMH, Hawes EM, Cooper JK, et al. Kinetics of oral trifluoperazine disposition in man. Br J Clin Pharmacol. 1983;15(3):380–2.
Verbeeck RK, Cardinal J-A, Hill AG, Midha KK. Binding of phenothiazine neuroleptics to plasma proteins. Biochem Pharmacol. 1983;32(17):2565–70.
Zhu X, Straubinger RM, Jusko WJ. Mechanism-based mathematical modeling of combined gemcitabine and birinapant in pancreatic cancer cells. J Pharmacokinet Pharmacodyn. 2015;42(5):477–96.
Avan A, Crea F, Paolicchi E, Funel N, Galvani E, Marquez VE, et al. Molecular mechanisms involved in the synergistic interaction of the EZH2 inhibitor 3-deazaneplanocin A with gemcitabine in pancreatic cancer cells. Mol Cancer Ther. 2012;11(8):1735–46.
Kroep JR, Giaccone G, Voorn DA, Smit EF, Beijnen JH, Rosing H, et al. Gemcitabine and paclitaxel: pharmacokinetic and pharmacodynamic interactions in patients with non-small-cell lung cancer. J Clin Oncol. 1999;17(7):2190–7.
Yilmaz B, Kadioglu YY, Aksoy Y. Investigation of the pharmacokinetics of gemcitabine and 2′,2′-difluorodeoxyuridine in human plasma by liquid chromatography. Anal Biochem. 2004;332(2):234–7.
Administration FaD. Gemcitabine injection [updated 8/2017 cited 2017 10/23]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209604s000lbl.pdf.
Esumi Y, Mitsugi K, Seki H, Takao A, Kawai M. Placental transfer, lacteal transfer and plasma protein binding of gemcitabine. Xenobiotica. 1994;24(10):957–64.
Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, Mitchell JB. Cytotoxic studies of paclitaxel (Taxol®) in human tumour cell lines. Br J Cancer. 1993;68(6):1104–9.
Administration FaD. Clinical pharmacology and biopharmaceutics review 2004 [updated 2004; cited 2017 10/23]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21660_ABRAXANE_biopharmr.PDF.
Administration FaD. ABRAXANE for injectable suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) [updated 01/07/2005; cited 2017 10/23]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021660lbl.pdf.
Skoda J, Hermanova M, Loja T, Nemec P, Neradil J, Karasek P, et al. Co-expression of cancer stem cell markers corresponds to a pro-tumorigenic expression profile in pancreatic adenocarcinoma. PLoS One. 2016;11(7):1–18.
Penchev VR, Rasheed ZA, Maitra A, Matsui W. Heterogeneity and targeting of pancreatic cancer stem cells. Clin Cancer Res. 2012;18(16):4277–84.
Lee G, Hall RR, Ahmed AU. Cancer stem cells: cellular plasticity, niche, and its clinical relevance. J Stem Cell Res Ther. 2016;06(10):1–20.
Breidert M, Keck T, Makowiec F, Lohrmann C, Harder J, Fischer R. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol. 2012;18(2):118–21.
Lonardo E, Cioffi M, Sancho P, Crusz S, Heeschen C. Studying pancreatic cancer stem cell characteristics for developing dew treatment strategies. J Vis Exp. 2015;100:1–9.
Vaz AP, Ponnusamy MP, Seshacharyulu P, Batra SK. A concise review on the current understanding of pancreatic cancer stem cells. J Cancer Stem Cell Res. 2014;2(4):1–12.
Rao CV, Mohammed A. New insights into pancreatic cancer stem cells. World J Stem Cells. 2015;7(3):547–55.
Zhu Y-Y, Yuan Z. Pancreatic cancer stem cells. Am J Cancer Res. 2015;5(3):894–906.
Rajeshkumar NV, Rasheed ZA, Garcia-Garcia E, Lopez-Rios F, Fujiwara K, Matsui WH, et al. A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol Cancer Ther. 2010;9(9):2582–92.
Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141(6):2218–27. e5
Kroep J, Giaccone G, Tolis C, Voorn D, Loves W, Van Groeningen C, et al. Sequence dependent effect of paclitaxel on gemcitabine metabolism in relation to cell cycle and cytotoxicity in non-small-cell lung cancer cell lines. Br J Cancer. 2000;83(8):1069–76.
Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2(3):260–9.
Shord SS, Patel SR. Paclitaxel alters the expression and specific activity of deoxycytidine kinase and cytidine deaminase in non-small cell lung cancer cell lines. J Exp Clin Cancer Res. 2009;28:76.
Shord SS, Camp JR, Young L. Paclitaxel decreases the accumulation of gemcitabine and its metabolites in human leukemia cells and primary cell cultures. Anticancer Res. 2005;25(6B):4165–72.
Shord SS, Faucette SR, Gillenwater HH, Pescatore SL, Hawke RL, Socinski MA, et al. Gemcitabine pharmacokinetics and interaction with paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2003;51(4):328–36.
Theodossiou C, Cook JA, Fisher J, Teague D, Liebmann JE, Russo A, et al. Interaction of gemcitabine with paclitaxel and cisplatin in human tumor cell lines. Int J Oncol. 1998;12(4):825–32.
Porter C, Waddell JA, Solimando JD. Cancer chemotherapy update: nab-paclitaxel plus gemcitabine regimen for pancreatic cancer. Hosp Pharm. 2014;49(1):18–22.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Lee JH, Kim SK, Khawar IA, Jeong SY, Chung S, Kuh HJ. Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma-mediated cell motility and drug resistance. J Exp Clin Cancer Res. 2018;37(1):4.
Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, et al. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano. 2015;9(4):3540–57.
Mato-Berciano A, Raimondi G, Maliandi MV, Alemany R, Montoliu L, Fillat C. A NOTCH-sensitive uPAR-regulated oncolytic adenovirus effectively suppresses pancreatic tumor growth and triggers synergistic anticancer effects with gemcitabine and nab-paclitaxel. Oncotarget. 2017;8(14):22700–15.
Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, et al. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335(1):41–51.
Zoli W, Ricotti L, Barzanti F, Dal Susino M, Frassineti GL, Milandri C, et al. Schedule-dependent interaction of doxorubicin, paclitaxel and gemcitabine in human breast cancer cell lines. Int J Cancer. 1999;80(3):413–6.
Miao X, Koch G, Straubinger RM, Jusko WJ. Pharmacodynamic modeling of combined chemotherapeutic effects predicts synergistic activity of gemcitabine and trabectedin in pancreatic cancer cells. Cancer Chemother Pharmacol. 2016;77(1):181–93.
Wicha SG, Chen C, Clewe O, Simonsson USH. A general pharmacodynamic interaction model identifies perpetrators and victims in drug interactions. Nat Commun. 2017;8(1):2129.
Pawaskar DK, Straubinger RM, Fetterly GJ, Hylander BH, Repasky EA, Ma WW, Jusko WJ. Synergistic interactions between sorafenib and everolimus in pancreatic cancer xenografts in mice. Cancer Chemother Pharmacol. 2013;71(5):1231–40.
Karlsson H, Fryknas M, Larsson R, Nygren P. Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exp Cell Res. 2012;318(13):1577–85.
Temraz S, Mukherji D, Alameddine R, Shamseddine A. Methods of overcoming treatment resistance in colorectal cancer. Crit Rev Oncol Hematol. 2014;89(2):217–30.
Ware MJ, Keshishian V, Law JJ, Ho JC, Favela CA, Rees P, et al. Generation of an in vitro 3D PDAC stroma rich spheroid model. Biomaterials. 2016;108:129–42.
Acknowledgments
This work was supported by Grant GM 24211 from the National Institutes of Health. We thank Dr. David D’Argenio for his help with ADAPT and Dr Gilbert Koch for advice on drug interaction modeling.
NOTE Graphs allowing rotation of Figures 2 to 5 can be found in the Supplementary Materials.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Figures
(PDF 156 kb)
Supplementary Tables
(PDF 87 kb)
ADAPT code
(PDF 65 kb)
Dataset for the three-drug interactions
(PDF 41 kb)
ESM 5
(GIF 1484 kb)
ESM 6
(GIF 1480 kb)
ESM 7
(GIF 1503 kb)
ESM 8
(GIF 1623 kb)
ESM 9
(GIF 1441 kb)
ESM 10
(GIF 1448 kb)
ESM 11
(GIF 1479 kb)
ESM 12
(GIF 1580 kb)
ESM 13
(GIF 1351 kb)
ESM 14
(GIF 1348 kb)
ESM 15
(GIF 1506 kb)
ESM 16
(GIF 1368 kb)
ESM 17
(GIF 870 kb)
ESM 18
(GIF 870 kb)
ESM 19
(GIF 871 kb)
ESM 20
(GIF 965 kb)
ESM 21
(GIF 882 kb)
ESM 22
(GIF 881 kb)
Rights and permissions
About this article
Cite this article
Molins, E.A.G., Jusko, W.J. Assessment of Three-Drug Combination Pharmacodynamic Interactions in Pancreatic Cancer Cells. AAPS J 20, 80 (2018). https://doi.org/10.1208/s12248-018-0235-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-018-0235-4