ABSTRACT
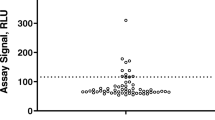
Biotherapeutics are known for their potential to induce drug specific immune responses, which are commonly evaluated by the detection of anti-drug antibodies (ADAs). For some biotherapeutics, pre-existing ADAs against drug have been observed in drug-naïve matrix. The presence of pre-existing drug specific antibodies may significantly complicate assessment of the screening ADA assay cutpoint value, which is usually established based on the statistical analysis of signal distribution from the drug-naïve individuals. A Gaussian mixture model-based approach is presented herein to address high prevalence of pre-existing ADAs to a modified monoclonal antibody-based biotherapeutic (m-mAb). A high prevalence of pre-existing anti-m-mAb antibodies was observed in drug-naïve individual cynomolgus monkey serum samples with signal ranging from 100 to 7000 relative light units (RLU, as determined in an electrochemiluminescence readout-based assay). Application of the industry standard statistical algorithm resulted in a relatively high floating screening assay cutpoint factor (CPF) of 9.80, which potentially would have reported a high percent of false negative samples. An alternative, Gaussian mixture model-based approach was applied to identify the least reactive individual samples in the tested population, which resulted in a floating screening assay CPF of 2.35. The low CPF value significantly reduced the risk of reporting false negative results. The proposed Gaussian mixture model-based approach described herein provides an alternate method for the calculation of biologically relevant screening assay CPF when high prevalence of pre-existing drug specific antibodies is observed.






Similar content being viewed by others
REFERENCES
Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48(5):1267–81.
Koren E, Smith HW, Shores E, Shankar G, Finco-Kent D, Rup B, et al. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J Immunol Methods. 2008;333(1–2):1–9.
Jani D, Marsden R, Mikulskis A, Gleason C, Klem T, Krinos Fiorotti C, et al. Recommendations for the development and validation of confirmatory anti-drug antibody assays. Bioanalysis. 2015;7(13):1619–31.
Hoffman D, Berger M. Statistical considerations for calculation of immunogenicity screening assay cut points. J Immunol Methods. 2011;373(1–2):200–8.
Jaki T, Lawo JP, Wolfsegger MJ, Singer J, Allacher P, Horling F. A formal comparison of different methods for establishing cut points to distinguish positive and negative samples in immunoassays. J Pharm Biomed Anal. 2011;55(5):1148–56.
Schlain B, Amaravadi L, Donley J, Wickramasekera A, Bennett D, Subramanyam M. A novel gamma-fitting statistical method for anti-drug antibody assays to establish assay cut points for data with non-normal distribution. J Immunol Methods. 2010;352(1–2):161–8.
Zhang L, Zhang JJ, Kubiak RJ, Yang H. Statistical methods and tool for cut point analysis in immunogenicity assays. J Immunol Methods. 2013;389(1–2):79–87.
Schaarschmidt F, Hofmann M, Jaki T, Grun B, Hothorn LA. Statistical approaches for the determination of cut points in anti-drug antibody bioassays. J Immunol Methods. 2015;418:84–100.
Xue L, Fiscella M, Rajadhyaksha M, Goyal J, Holland C, Gorovits B, et al. Pre-existing biotherapeutic-reactive antibodies: survey results within the American Association of Pharmaceutical Scientists. AAPS J. 2013;15(3):852–5.
Gorovits B, Clements-Egan A, Birchler M, Liang M, Myler H, Peng K, et al. Pre-existing antibody: biotherapeutic modality-based review. AAPS J. 2016;18(2):311–20.
FDA. Guidance for industry assay development for immunogenicity testing of therapeutic proteins draft guideline. 2009.
Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53(5):793–808.
Mikulskis A, Yeung D, Chen W, Mehta D, Amaravadi L. Novel data analysis methods to overcome cut point challenges and enable comprehensive assessment of antidrug binding activity in confirmatory assays. J Immunol Methods. 2013;392(1–2):38–48.
ACKNOWLEDGMENTS
The authors would like to thank Joanne Wentland for her bioanalytical contribution in characterizing the nature of the pre-existing antibodies as well as Frank Barletta and Beth Leary for their helpful comments and suggestions on the manuscript. The funding for the presented research and for the manuscript preparation was provided by Pfizer Inc. (MA, USA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Seema C Kumar and Jason A DelCarpini contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kumar, S.C., DelCarpini, J.A., Qu, Q. et al. Mitigation of Pre-existing Antibodies to a Biotherapeutic in Non-clinical Species When Establishing Anti-drug Antibody Assay Cutpoint. AAPS J 19, 313–319 (2017). https://doi.org/10.1208/s12248-016-0011-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-0011-2