Abstract
Introduction
Ventilator-associated pneumonia (VAP) is reported as the second most common nosocomial infection among critically ill patients with the incidence ranging from 2 to 16 episodes per 1000 ventilator days. The use of probiotics has been shown to have a promising effect in many RCTs. Our systematic review and meta-analysis were thus planned to determine the effect of probiotic use in critically ill ventilated adult patients on the incidence of VAP, length of hospital stay, length of ICU stay, duration of mechanical ventilation, the incidence of diarrhea, and the incidence of oropharyngeal colonization and in-hospital mortality.
Methodology
Systematic search of various databases (such as Embase, Cochrane, and Pubmed), published journals, clinical trials, and abstracts of the various major conferences were made to obtain the RCTs which compare probiotics with placebo for VAP prevention. The results were expressed as risk ratios or mean differences. Data synthesis was done using statistical software - Review Manager (RevMan) Version 5.4 (The Cochrane Collaboration, 2020).
Results
Nine studies met our inclusion criterion and were included in the meta-analysis. The incidence of VAP (risk ratio: 0.70, CI 0.56, 0.88; P = 0.002; I2 = 37%), duration of mechanical ventilation (mean difference −3.75, CI −6.93, −0.58; P 0.02; I2 = 96%), length of ICU stay (mean difference −4.20, CI −6.73, −1.66; P = 0.001; I2 = 84%) and in-hospital mortality (OR 0.73, CI 0.54, 0.98; P = 0.04; I2 = 0%) in the probiotic group was significantly lower than that in the control group. Probiotic administration was not associated with a statistically significant reduction in length of hospital stay (MD −1.94, CI −7.17, 3.28; P = 0.47; I2 = 88%), incidence of oro-pharyngeal colonization (OR 0.59, CI 0.33, 1.04; P = 0.07; I2 = 69%), and incidence of diarrhea (OR 0.59, CI 0.34, 1.03; P = 0.06; I2 = 38%).
Discussion
Our meta-analysis shows that probiotic administration has a promising role in lowering the incidence of VAP, the duration of mechanical ventilation, length of ICU stay, and in-hospital mortality.
Similar content being viewed by others
Background
Ventilator-associated pneumonia (VAP) is reported as the second most common nosocomial infection among critically ill patients [1] with the incidence ranging from 2 to 16 episodes per 1000 ventilator days [2]. VAP is associated with an increase in the duration of hospitalization by 7 days, an increase in the healthcare cost by approximately 40,000 USD [3] and is reported to be the leading cause of death among nosocomial infections [4]. The pathogenesis of VAP is very complex but primarily involves bacterial translocation and colonization of the aerodigestive tract with pathogenic bacteria. This is followed by aspiration of these pathogenic micro-organisms into the lower respiratory tract thus causing pneumonia [5].
Numerous trials and studies are done to determine the best pharmacological preventive strategies inhibiting the colonization of the micro-organisms such as the use of antibiotics for selective digestive decontamination (SDD) or selective oral decontamination (SOD) or the use of probiotics. The use of antibiotics for SDD or SOD has been associated with an increase in antibiotic resistance and cost [6] but the use of probiotics as a preventive measure has been shown to have promising results in various studies [7]. Probiotics are live non-pathogenic microbes that reduce bacterial translocation by activating mucosal immunity and regulating the release of proinflammatory cytokines [5]. They also inhibit the growth of pathogenic micro-organisms by many mechanisms which include the production of various substances (such as organic acid, hydrogen peroxide, and bacteriocins), competition for nutrients, inhibition of pathogen attachment, and inhibition of the action of microbial toxins. Probiotics also stimulate the proliferation of the normal epithelium which helps maintain the mucosal defense barrier [8]. Prebiotics are non-digestible sugars that selectively stimulate the growth of certain bacterial colonies while a combination of probiotics and prebiotics is called synbiotics [9].
Seeing the promising nature of probiotics in critically ill patients, many randomized controlled trials (RCTs) have been conducted in recent years to evaluate the effectiveness of probiotics in the prevention of VAP. A recent meta-analysis performed by Su et al. in 2020 [1] and Cheng et al. in 2018 [5] showed that probiotics are efficient in decreasing the incidence of VAP. However, the meta-analysis by Su et al. included few studies [10, 11] in which the use of probiotics was not compared with placebo as a control. Also, the trials included in the study [12, 13] were of low quality. A large meta-analysis with trial sequential analysis published by Weng et al. [14] in 2017 also supported the role of probiotics in the prevention of VAP but the study included children in the patient population.
Thus, the current study was planned to determine the effect of the use of probiotics in critically ill ventilated adult patients on the incidence of VAP, length of hospital stay, length of ICU stay, duration of mechanical ventilation, the incidence of diarrhea, and the incidence of oropharyngeal colonization and in-hospital mortality.
Methodology
Protocol preparation and registration
A protocol for the study was prepared and has been registered in Prospero [15].
Eligibility criteria
The protocol was prepared to include RCTs done on critically ill patients on a ventilator. RCTs selected were those which used probiotics/synbiotics in the patients of the intervention arm and used placebo in the control arm patients. Studies using any other medication besides the placebo in the control arm were not included in the study as it would have led to bias in the study.
Data sources
Two reviewers independently made a systematic search of EMBASE, MEDLINE (Pubmed), Web of Science, and the Cochrane Central Registry of Controlled Trials (CENTRAL) from inception to February 2020, to include clinical trials conducted in humans regarding probiotics and VAP. The search was limited to studies published in English. Search terms included “critically ill” “sepsis” “trauma” “ventilation- associated” “probiotics” “synbiotics”. Abstracts of major conferences and trials database were also searched for. Bibliographies of all relevant trials, systematic reviews, and meta-analysis were also hand-scanned.
Study selection
Two reviewers (PB and KDS) independently screened studies for inclusion depending on the eligibility criterion. Randomized control trials reporting the use of probiotics for the prevention of ventilator-associated pneumonia (VAP) in critically ill patients admitted in intensive care units (ICUs) were included in the meta-analysis. Studies reporting different types of probiotics (Lactobacillus spp., Pediococcus spp., Leuconostoc spp., Bifidobacterium spp., Bacillus subtilis, Streptococcus spp., Ergyphilus spp., Bifidus spp., Saccharomyces spp., Enterococcus spp.) alone or in combination with prebiotics were included in the study. Studies including pediatric patients or studies using probiotics as therapeutic agents or studies comparing large versus low doses of probiotics or studies comparing different types of probiotics were excluded from our study.
Data extraction
Both authors (PB and KDS) screened and evaluated titles and available abstracts of identified citations in duplicate to determine eligibility. Full-text publication of all articles that were judged as potentially eligible by the review team was downloaded and eligibility criteria were applied to the full text of all potentially eligible trials. Any disagreement between the reviewers was resolved by consensus and any discrepancy remaining was further resolved through discussion with the arbitrator third author (PM). The Phi or kappa statistics were applied to measure the interobserver agreement regarding the eligibility of the RCTs.
Standardized form from the Cochrane Data Collection template was adapted and used to create a study-specific data abstraction form. Two reviewers (PB and KDS) extracted the data, independently and in duplicate, from all eligible studies.
Data items
Data abstracted included demographic information, methodology, intervention details, and outcome data. The primary outcome to be studied was the incidence of VAP. Secondary outcomes that were studied included duration of mechanical ventilation, length of hospital or ICU stay (as reported), oropharyngeal colonization, the incidence of diarrhea, and mortality rate (ICU/in-hospital mortality) as reported in the study.
Risk of bias assessment
Risk of bias was assessed by reviewers using a modified plausible quality assessment scale as recommended by the Cochrane Collaboration. This instrument included response options of “low,” “high,” or “unclear” risk of bias.
The key domains that were evaluated included random sequence generation; allocation concealment; blinding of participants/healthcare professionals/data collectors/outcome assessors/data analysts; incomplete outcome data; and reviewer’s bias. Reviewers resolved disagreement by discussion and the arbitrator adjudicated any unresolved disagreements.
Summary measures
The incidence of ventilator-associated pneumonia was measured using risk ratio; the incidence of oro-pharyngeal colonization, the incidence of diarrhea, and in-hospital mortality were measured using odds ratio; and duration of mechanical ventilation, length of ICU stay, and length of hospital stay were measured using differences in means.
Strategy for data synthesis
Random effect meta-analyses were used to compare similar interventions with high heterogeneity. Random effect meta-analyses included both within and between-study differences. If heterogeneity was lower, then the fixed effect meta-analyses were applied. Heterogeneity of treatment effect was assessed using Cochrane’s Q statistic and I squared statistic. Excess heterogeneity was explained using multiple approaches such as subgroup effect or sensitivity analyses. Dichotomous outcomes were reported using relative risk ratio (RR) or odds (OR) ratio whereas continuous endpoints reported in trials were calculated as weighted mean difference (MD) and standard deviation (SD). The inverse of variance was used to provide individual weightage to the studies.
Subgroups analysis
Following subgroup analysis was performed for the primary outcome that is the incidence of VAP to explain the heterogeneity found in the studies.
Subgroup analysis of the trial grouping based on the risk of bias was done, i.e., high-risk trials vs low-risk trials.
Analysis of trials for the primary outcome for reporting in specific populations such as trauma, medical, or surgical patients.
Subgroup analysis of trials reporting micro-organisms with the trials not reporting microorganisms specifically for causation of VAP.
Sensitivity analysis
In few studies, the duration of mechanical ventilation [16, 17], length of ICU stay [16,17,18], and length of hospital stay [17] were given as median (IQR) which was converted to mean ± SD for inclusion in the meta-analysis to maintain uniformity of the study results. Sensitivity analysis was done by the removal of these studies to see the change in the incidence of VAP upon removal of the concerned studies.
Results
Study identification and selection
A systematic search of the database was made using the keywords which gave a total of 299 articles. By manual search of the references of systematic reviews and meta-analysis, 17 additional records were found. After removal of the duplicate articles, titles and abstracts of 274 publications were searched. Of these, 246 records could be easily excluded as they did not meet our inclusion criteria being animal studies (n = 7) or children studies (n = 43) or being done on patients who were not critically ill (n = 34). The reasons for exclusion are enlisted in Fig. 1. Full text of the remaining 28 articles was obtained and only 9 of these were found eligible for quantitative synthesis in our meta-analysis. The remaining 19 articles could be excluded as one of these was a cohort study; in 15, the outcome assessed was not VAP, in one, it was found that the study was conducted in medical wards, and in 2 of these placebos was not used in the control group. Instead, antibiotic decontamination or chlorhexidine mouth wash was being used. There was 96% agreement (Cohen’s k 0.92) between the two authors (PB and KDS).
Characteristics of the included study
The characteristics of the nine studies included in the meta-analysis are given in Table 1. The table gives a detailed description of all the included studies in terms of the study design, duration of follow-up, patient population under investigation and its characteristics, intervention done, control group, outcome measured, and the definition of VAP used in the study. Most of the included studies were published in the past 10 years and their mean sample size was 125 (ranging from 52 to 259). Of the nine studies included, all [7, 16,17,18,19,20,21,22,23] the studies reported VAP in the included patients; 8 studies described length of ICU stay [7, 16,17,18,19, 21,22,23] and in-hospital mortality [7, 16,17,18,19,20,21,22]; 5 studies described duration of mechanical ventilation [7, 16, 17, 21, 22]; only 4 studies described the length of hospital stay [7, 17, 19, 22] and incidence of diarrhea [7, 19, 21, 22] and incidence of oro-pharyngeal colonization [7, 16, 17, 22]. The heterogeneity of the studies included was assessed using the Cochrane Q statistics.
Probiotics administered including the dosage and routes of administration varied in the studies. In one of the studies, a single probiotic (Lactobacillus rhamnosus [7]) was used, 5 studies used multiple probiotics [16, 18, 19, 22, 23], and in 3 studies a symbiotic formula (Synbiotic 2000 Forte) [17, 20, 21] was used. The severity of illness of the patients included in the study is also provided in the table.
There was variability in the definition of VAP among all studies as shown in Table 1. In two studies [7, 16] both clinical and microbiological definition was mentioned while in one study [18] no explicit definition of VAP was given. The outcome data extracted from the RCTs included in the meta-analysis are presented in Table 1.
Risk of bias assessment
The risk of bias in the included studies is shown in Figs. 2 and 3. All the nine studies had a low risk of random sequence generation selection bias, allocation concealment selection bias, and selective reporting bias. Two studies had a high risk of performance bias and detection bias as they did not have good blinding of participants and personnel. The risk of selection and reporting bias was low in all the studies. However, the risk of outcome assessment detection bias was high in most of the studies. The presence of detection bias can either underestimate or overestimate the size of the effect.
Primary outcome: incidence of VAP
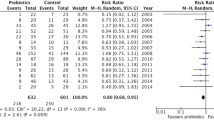
All the nine RCTs included in the study with a total patient load of 1127 (564 in probiotics group and 563 in the placebo group) reported VAP incidence as can be seen in Fig. 4. The analysis showed that the incidence of VAP in the probiotic group was significantly lower than the incidence in the control group (OR 0.70, CI 0.56, 0.88; P = 0.002; I2 = 37%). Low to moderate heterogeneity was seen between studies.
Secondary outcome
The other outcomes measured duration of mechanical ventilation, length of ICU stay, length of hospital stay, the incidence of oropharyngeal colonization, the incidence of diarrhea, and in-hospital mortality. The duration of mechanical ventilation, length of ICU stay and length of hospital stay were reported either in mean ± SD or median (IQR). For comparison, all the results were taken in mean ± SD. The conversion of the median (IQR) to mean ± SD was done using the following formula [24].
Mean = (a + b + 2m)/4; where a is the low range; b is the high range; m is the median
Variance (S2) = 1/12 {[(a−2m + b)2/4] + (b−a)2}
Duration of mechanical ventilation
Five of the nine studies with a total patient size of 799 patients (399 in probiotics and 400 in the placebo arm) provided the duration of mechanical ventilation (Fig. 5). A high heterogeneity (MD −3.75, CI −6.93, −0.58; P = 0.02; I2 = 96%) was seen between the studies. There was a statistically significant reduction in the duration of mechanical ventilation in the probiotic group. In two studies [16, 17], the duration of mechanical ventilation was expressed in the median (IQR) and was converted into mean ± SD. If we remove both these studies for sensitivity analysis, the mean difference becomes statistically non-significant = −4.32 (−9.12, 0.49, P = 0.08).
Length of ICU stay
Eight of the studies reported length of ICU stay in 1072 patients (538 in the probiotic arm and 534 in the placebo arm) as seen in Fig. 6. A high heterogeneity (MD −4.20, CI −6.73, −1.66; P = 0.001; I2 = 84%) was seen between the studies. In three studies [16,17,18], the length of ICU stay was expressed in median (IQR) and was converted into mean ± SD. If we remove these three studies for sensitivity analysis, the mean difference still remains statistically significant −4.37 (−7.89, −0.85; P = 0.01).
Length of hospital stay
Four of the studies reported length of hospital stay in 648 patients (324 in the probiotic arm and 324 in the placebo arm) as seen in Fig. 7. A high heterogeneity (MD −1.94, CI −7.17, 3.28; P = 0.47; I2 = 88%) was seen between the studies. In one study [17], the length of hospital stay was expressed in median (IQR) and was converted into mean ± SD. If we remove this study, the mean difference remains non-significant = −3.79 (−8.47, 0.89; P = 0.11).
Incidence of oropharyngeal colonization
Four of the studies reported oropharyngeal colonization in 674 patients (332 in the probiotic arm and 342 in the placebo arm) as seen in Fig. 8. A high heterogeneity (OR 0.59, CI 0.33, 1.04; P = 0.07; I2 = 69%) was seen between the studies.
Incidence of diarrhea
Four of the studies reported diarrhea in 454 patients (229 in the probiotic arm and 225 in the placebo arm) as seen in Fig. 9. A moderate heterogeneity (OR 0.59, CI 0.34, 1.03; P = 0.06; I2 = 38%) was seen between the studies.
In-hospital mortality
Eight of the studies reported in-hospital mortality in 1086 patients (542 in the probiotic arm and 544 in the placebo arm) as shown in Fig. 10. No heterogeneity (OR 0.73, CI 0.54, 0.98; P = 0.04; I2 = 0%) was seen between the studies and a statistically significant difference was seen.
Subgroup analysis
High vs low risk of bias trials
The incidence of VAP was statistically significant in trials reporting high risk of bias (RR 0.59, CI 0.38, 0.92; P = 0.02; I2 = 47%) while it was not significant in those reporting low risk of bias (RR 0.76, CI 0.57, 1.02; P = 0.07; I2 = 40%). However, the overall test for subgroup differences was not found to be statistically significant (P = 0.35), as can be seen in Fig. 11.
Mixed population vs trauma population trials
The incidence of VAP was similar in trials done in a mixed population of patients (RR 0.67, CI 0.46, 0.96; P = 0.03; I2 = 56%) as well as those done in the trauma population (RR 0.73, CI 0.56, 0.95; P = 0.02; I2 = 0%). The difference between the subgroups was not statistically significant (P = 0.70) as can be seen in Fig. 12.
Trials reporting micro-organisms vs not reporting micro-organisms
The incidence of VAP was similar in trials reporting microorganisms (RR 0.69, CI 0.56, 0.85; P = 0.0005; I2 = 0%) as well as those not reporting microorganisms (RR 0.70, CI 0.34, 1.42; P = 0.32; I2 = 79%). The difference between the subgroups was not statistically significant (P = 0.99) as can be seen in Fig. 13.
Publication bias
A funnel plot was drawn for the primary outcome that is the incidence of VAP to determine the presence of possible publication bias. As can be seen in Fig. 14, there was no apparent publication bias as the funnel plot is symmetrical.
Discussion
The current meta-analysis was planned to determine the effect of probiotics in the prevention of VAP by including randomized control trials on adults as patient populations. A total of nine studies were included in the meta-analysis and most of these studies had a low risk of selection, performance, reporting, and attrition bias. Few of the studies had a high detection bias risk. The forest plot analysis of the outcomes showed that probiotics had a good effect in reducing the incidence of VAP (P = 0.002), the duration of mechanical ventilation (P = 0.02), length of ICU stay (P = 0.001), and in-hospital mortality (P = 0.04). However, the use of probiotics did not affect the length of hospital stay (P = 0.47), the incidence of oropharyngeal colonization (P = 0.07), and the incidence of diarrhea (P = 0.06).
During the review, few assumptions were made as studies were inconsistent in reporting measures of association and differed in characteristics and timelines of endpoints. These assumptions were mostly related to the secondary outcomes of the review. We had used an empiric conversion for converting median reported values to mean using the equation. We did a sensitivity analysis excluding the studies for which this was done. We found that magnitude of effect size changes for the outcomes; duration of mechanical ventilation becomes non-significant from significant (−4.32 [−9.12, 0.49]) (P = 0.08) while the length of ICU stay, the mean difference still remained significant (−4.37 [−7.89, −0.85]) (P = 0.01). However, the direction of effect remained unaltered. The length of hospital stay remained non-significant −3.79 (−8.47.89; P = 0.11) on sensitivity analysis. Similar assumptions and its sensitivity analysis were not made in any of the previous meta-analysis.
We did subgroup analysis based on prior assumptions that effect estimates of probiotics may vary based on the quality of trials, population characteristics, and reporting of microorganism. However, we did not find evidence of interaction between postulated subgroups, and differences between the subgroups were non-significant implying the overall effect size estimates were consistent between subgroups both quantitatively and qualitatively. Bo et al. [25] in their meta-analysis also showed that even after the removal of studies with a high risk of bias probiotics still had a positive effect on the incidence of VAP.
A recent meta-analysis by Su et al. [26] published in 2020, showed that probiotic administration was associated with a statistically significant reduction in the incidence of VAP and a reduction in the duration of antibiotic use for VAP. However, two studies included in the meta-analysis [10, 11] did not compare the use of probiotics with placebo. The study by Oudhuis et al. [10] compared antibiotic use with probiotics in the reduction of VAP rate while the study by Klarin et al. [11] compared the use of probiotics with chlorhexidine mouth wash. Also, two studies included in the meta-analysis-Spindler Vessel et al. [12] and Forestier et al. [13] reported pneumonia which may not be ventilator associated. Thus, the above meta-analysis may not be determining the effect of probiotics on VAP accurately. For our meta-analysis, the protocol and trial of one large multicenter study by Deborah Cook et al. [27] were found eligible. Since the results were not published, the authors were mailed to share the results, but the same was not shared.
Our meta-analysis shows that the administration of probiotics significantly decreases the incidence of VAP, the duration of mechanical ventilation, length of ICU stay, and in-hospital mortality compared to placebo. The decrease in the incidence of VAP after probiotic administration is consistent with the previous meta-analysis by Su et al. [26], Weng et al. [14], Chen et al. [5], Liu et al. [28], Manzanares et al. [29], Siempos et al. [30], Bo et al. [8], and Banupriya et al. [31]. However, two meta-analyses, Gu et al. [32] and Wang et al. [33] did not show a statistically significant decrease in the incidence of VAP after probiotic administration.
In our study, no statistically significant decrease was seen in the length of hospital stay, the incidence of diarrhea, and the incidence of oropharyngeal colonization. However, a statistically significant reduction in the duration of mechanical ventilation, length of ICU stay, and in-hospital mortality was seen in our study which was not reported in other meta-analyses [5, 14, 25, 26, 28,29,30,31,32,33]. A meta-analysis conducted by Siempos et al. in 2010 [30] showed a reduction in length of ICU stay and respiratory tract colonization by Pseudomonas aeruginosa. Gu et al. in 2014 [34] also showed a reduction in length of ICU stay with the administration of probiotics. However, the meta-analysis by Siempos et al. [30] is old and new RCTs have been reported after that. The meta-analysis by Gu et al. in 2014 [34], described the length of ICU stay in only two studies which is a statistically insignificant number.
The absence of any effect on the other secondary outcomes in our meta-analysis could be due to the variability in the populations studied, the probiotic agents used, doses, time points when therapy was initiated, durations of therapy, the routes of administration, and the diagnostic criteria used for establishing VAP.
The definition of VAP used in the included RCTs was variable. In two of the RCTs [7, 16], two VAP rates were given, microbiological as well as clinical VAP. Of the two, the microbiological definition of VAP was used for our meta-analysis, as this is the definition used most consistently by many authors in various RCTs/meta-analysis. Thus, VAP definition is an important limitation of our meta-analysis as we relied on the reported definitions; a uniform definition is lacking in the RCTs. Large multicentric RCT with a uniform objective definition of ventilator-associated event (VAE) needs to be done in the future to precisely evaluate the effect of probiotics on VAP. VAE, as defined by CDC, is said to happen if after a period of stability or improvement, the patient has worsening oxygenation (minimum FiO2 increases by ≥ 0.2 or minimum daily PEEP increases by ≥ 3 cm H2O) [35].
To calculate for the incidence of oropharyngeal colonization, the rate of colonization at day 7 of ICU stay was selected. The definition of diarrhea as defined in most studies was ≥ 3 liquid stools/day [7, 19, 22].
There are few limitations of the meta-analysis. Firstly, the type, duration, and mode of administration of probiotics in the various RCTs were not constant among the various RCTs. The treatment duration in few studies was too short for any concrete evidence. Secondly, the diagnosis of ventilator-associated pneumonia was based on varied definitions in the RCTs (as listed in the table) with the element of subjectivity. Though the recent CDC definition of VAE is more objective, but, it is not yet used by any of the published RCTs on probiotics.
Thirdly, we were unable to assess the impact of probiotics on other clinically important endpoints: length of antibiotic therapy and antibiotic consumption. This is because of sparse and inconsistent reporting of the above endpoints across trials. Fourth, RCTs included in the meta-analysis have excluded immunocompromised patients. Thus, the role of probiotics in this important patient population cannot be ascertained. Furthermore, no study reported any side effects of probiotics use.
Thus, a large, multicentric, randomized control trial evaluating the use of probiotics (optimal type, dose, and route of administration) for VAP in an immunocompromised patient population is needed which should also evaluate the possible side effects of probiotics. The trials can also evaluate the changes in the microbiome following critical illness and the effect of probiotics/synbiotics on restoring a healthy microbiome in treated patients.
The strength of this current systematic review includes the use of standard methods to reduce bias (comprehensive literature search, duplicate data abstraction, specific criteria for searching and analysis), and the analysis of relevant clinical outcomes in the critically ill. Additional conduct of explicit subgroup and sensitivity analysis provides evidence in the robustness of estimates.
Conclusion
It can be concluded that the use of probiotics reduces the incidence of VAP, duration of mechanical ventilation, length of ICU stay, and in-hospital mortality but has no effect on the length of hospital stay, incidence of diarrhea, and incidence of oropharyngeal colonization. The benefit of probiotics seems clinically plausible, as the effect estimates were favoring probiotics in most above clinically related endpoints. However, the varying definitions and subjectivity of VAP criteria preclude true estimates of effect. An objective uniform definition of VAP and large scale and large multicentric randomized controlled trials are needed to evaluate the further optimal type, dose, and route of administration for probiotics in ventilator association pneumonia.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- VAP:
-
Ventilator-associated pneumonia
- ICU:
-
Intensive care unit
- USD:
-
Unites States dollar
- SDD:
-
Selective digestive decontamination
- SOD:
-
Selective oral decontamination
- RCT:
-
Randomized controlled trial
- PB:
-
Priyam Batra
- KDS:
-
Kapil Dev Soni
- PM:
-
Purva Mathur
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- CDC:
-
Center for disease control and prevention
References
Timsit J-F, Esaied W, Neuville M, Bouadma L, Mourvillier B. Update on ventilator-associated pneumonia. F1000Research. 2017;6 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5710313/. Cited 2020 Feb 17.
International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. - PubMed - NCBI [Internet]. [cited 2020 Feb 17]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21908073/.
Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31(5):1312–7.
Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospitalacquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. 2017;50(3):1700582.
Chen C, Wang J, Yin M, Zhao Q. Probiotics are effective in decreasing the incidence of ventilator-associated pneumonia in adult patients: a meta-analysis of randomized controlled trials. :9.
Decontamination of the Digestive Tract and Oropharynx in ICU Patients | NEJM [Internet]. [cited 2020 Feb 19]. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa0800394.
Morrow LE, Kollef MH, Casale TB. Probiotic Prophylaxis of Ventilator-associated Pneumonia: A Blinded, Randomized, Controlled Trial. Am J Respir Crit Care Med. 2010;182(8):1058–64.
Singhi SC, Kumar S. Probiotics in critically ill children. F1000Research. 2016;5:407.
Pandey Kavita R, Naik Suresh R, Vakil Babu V. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol. 2015 Dec;52(12):7577–87.
Su M, Jia Y, Li Y, Zhou D, Jia J. Probiotics for the Prevention of Ventilator-Associated Pneumonia: A Meta-Analysis of Randomized Controlled Trials. Respir Care. 2020:respcare.07097.
Oudhuis GJ, Bergmans DC, Dormans T, Zwaveling J-H, Kessels A, Prins MH, et al. Probiotics versus antibiotic decontamination of the digestive tract: infection and mortality. Intensive Care Med. 2011 Jan;37(1):110–7.
Klarin B, Adolfsson A, Torstensson A, Larsson A. Can probiotics be an alternative to chlorhexidine for oral care in the mechanically ventilated patient? A multicentre, prospective, randomised controlled open trial. Crit Care Lond Engl. 2018;22(1):272.
Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr. 2007;31(2):119–26.
Forestier C, Guelon D, Cluytens V, Gillart T, Sirot J, De Champs C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebocontrolled pilot study in intensive care unit patients. Crit Care. 2008;12(3):R69.
Weng H, Li J-G, Mao Z, Feng Y, Wang C-Y, Ren X-Q, et al. Probiotics for Preventing Ventilator-Associated Pneumonia in Mechanically Ventilated Patients: A Meta-Analysis with Trial Sequential Analysis. Front Pharmacol. 2017;8:717.
PROSPERO email history [Internet]. [cited 2020 Jun 19]. Available from: https://www.crd.york.ac.uk/prospero/record_email.php.
Zeng J, Wang C-T, Zhang F-S, Qi F, Wang S-F, Ma S, et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42(6):1018–28.
Knight DJW, Gardiner D, Banks A, Snape SE, Weston VC, Bengmark S, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009;35(5):854–61.
Shimizu K, Yamada T, Ogura H, Mohri T, Kiguchi T, Fujimi S, et al. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: a randomized controlled trial. Crit Care. 2018;22(1):239.
Barraud D, Blard C, Hein F, Marçon O, Cravoisy A, Nace L, et al. Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med. 2010;36(9):1540–7.
Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro- and Synbiotics to Control Inflammation and Infection in Patients With Multiple Injuries. J Trauma Inj Infect Crit Care. 2009;67(4):815–21.
Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a Synbiotic Formula (Synbiotic 2000Forte®) in Critically Ill Trauma Patients: Early Results of a Randomized Controlled Trial. World J Surg. 2006;30(10):1848–55.
Mahmoodpoor A, Hamishehkar H, Asghari R, Abri R, Shadvar K, Sanaie S. Effect of a Probiotic Preparation on Ventilator-Associated Pneumonia in Critically Ill Patients Admitted to the Intensive Care Unit: A Prospective Double-Blind Randomized Controlled Trial. Nutr Clin Pract. 2019;34(1):156–62.
Tan M, Zhu J-C, Du J, Zhang L-M, Yin H-H. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290.
Median/Range [Internet]. [cited 2020 Jun 6]. Available from: http://vassarstats.net/median_range.html.
Bo L, Li J, Tao T, Bai Y, Ye X, Hotchkiss RS, et al. Probiotics for preventing ventilatorassociated pneumonia. Cochrane Database Syst Rev. 2014; Available from: http://doi.wiley.com/10.1002/14651858.CD009066.pub2. Cochrane Acute Respiratory Infections Group, editor. Cited 2020 Feb 16.
Cook DJ, Johnstone J, Marshall JC, Lauzier F, Thabane L, Mehta S, et al. Probiotics: Prevention of Severe Pneumonia and Endotracheal Colonization Trial-PROSPECT: a pilot trial. Trials. 2016;02(17):377.
Liu K, Zhu Y, Zhang J, Tao L, Lee J-W, Wang X, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16(3):R109.
Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;20(1):262.
Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: A meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(3):954–62.
Banupriya B, Biswal N, Srinivasaraghavan R, Narayanan P, Mandal J. Probiotic prophylaxis to prevent ventilator associated pneumonia (VAP) in children on mechanical ventilation: an openlabel randomized controlled trial. Intensive Care Med. 2015;41(4):677–85.
Gu W-J, Wei C-Y, Yin R-X. Lack of Efficacy of Probiotics in Preventing Ventilator-Associated Pneumonia. Chest. 2012;142(4):859–68.
Wang J, Liu K, Ariani F, Tao L, Zhang J, Qu J-M. Probiotics for Preventing Ventilator-Associated Pneumonia: A Systematic Review and Meta-Analysis of High-Quality Randomized Controlled Trials. PLoS One. 2013;8(12):e83934 Salluh JI, editor.
Gu W-J, Deng T, Gong Y-Z, Jing R, Liu J-C. The Effects of Probiotics in Early Enteral Nutrition on the Outcomes of Trauma: A Meta-Analysis of Randomized Controlled Trials. :9.
Ventilator-Associated Event (VAE). 2020;49.
Acknowledgements
We would like to acknowledge the administration of AIIMS, New Delhi, for giving all the necessary resources for the data collection and interpretation.
Funding
No funding was obtained from any source in designing/conducting/publishing the study.
Author information
Authors and Affiliations
Contributions
All three authors have contributed substantially in the conception, designing, data collection, interpretation, and preparation of the manuscript. The authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Batra, P., Soni, K.D. & Mathur, P. Efficacy of probiotics in the prevention of VAP in critically ill ICU patients: an updated systematic review and meta-analysis of randomized control trials. j intensive care 8, 81 (2020). https://doi.org/10.1186/s40560-020-00487-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-020-00487-8