Abstract
Background
The 2010 Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) guidelines are the only Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines for cow’s milk allergy (CMA). They indicate oral food challenge (OFC) as the reference test for diagnosis, and suggest the choice of specific alternative formula in different clinical conditions. Their recommendations are flexible, both in diagnosis and in treatment.
Objectives & methods
Using the Scopus citation records, we evaluated the influence of the DRACMA guidelines on milk allergy literature. We also reviewed their impact on successive food allergy and CMA guidelines at national and international level. We describe some economic consequences of their application.
Results
DRACMA are the most cited CMA guidelines, and the second cited guidelines on food allergy. Many subsequent guidelines took stock of DRACMA’s metanalyses adapting recommendations to the local context. Some of these chose not to consider OFC as an absolute requirement for the diagnosis of CMA. Studies on their implementation show that in this case, the treatment costs may increase and there is a risk of overdiagnosis. Interestingly, we observed a reduction in the cost of alternative formulas following the publication of the DRACMA guidelines.
Conclusions
DRACMA reconciled international differences in the diagnosis and management of CMA. They promoted a cultural debate, improved clinician’s knowledge of CMA, improved the quality of diagnosis and care, reduced inappropriate practices, fostered the efficient use of resources, empowered patients, and influenced some public policies. The accruing evidence on diagnosis and treatment of CMA necessitates their update in the near future.
Similar content being viewed by others
Background
The mission of the World Allergy Organization (WAO) is to advance excellence in clinical care, research, education and training. Clinical practice guidelines are part of this mission. In the last 10 years, WAO produced guidelines on anaphylaxis, allergy prevention, urticaria, allergy training, hereditary angioedema, and molecular diagnosis [1]. All of these documents aim at deepening the clinician’s knowledge, improving the quality of diagnosis and care and reducing inappropriate variation in practice. Application of these guidelines may promote the efficient use of resources, inform and empower patients and support public policies [2]. However, their introduction into routine daily practice requires a series of educational, social and political steps. If not correctly implemented, the guidelines may fail their objectives and patients may remain exposed to harmful or unnecessary care [3]. Barriers to guideline implementation may be encountered at different levels [4]:
-
Individual, as professionals may have difficulty understanding the guidelines’ language; they may also introduce personal bias in thinking, balancing benefits and risks, and reaching different conclusions;
-
Motivational, as different factors/barriers may generate different motivational stages in individual professionals;
-
Relating to organizational context, for instance lack of arrangements for continuous learning, and lack of implementation tools;
-
Social, for the interference of existing values and cultures, and for the influence of the opinion of key people;
-
Economic, for insufficient or no reimbursement arrangements, rewards, health care systems or incentives.
To overcome these limitations, a series of educational tools needs to be put into play. In this article, we will evaluate the impact on real life of DRACMA, the GRADE guidelines on diagnosis and treatment of CMA [5], and their dissemination.
DRACMA’s influence on the subsequent literature
The original version, published in the World Allergy Organization Journal (WAO Journal), was co-published in Pediatric Allergy and Immunology (PAI) [6]. In 2011, DRACMA was the most downloaded article from PAI website, the second in 2012 and the third in 2013. The publication in the WAO Journal was the most accessed article in 2011 and 2012. The last available data (up to 2015) still indicate that it ranks in the top ten. Up to August 15, 2017, according the Scopus data, 241 articles cited the two versions. A summary report was published at the end of 2010 [7]. As for mid-august 2017, it has been cited 109 times thus far, with a 6.57 Field-Weighted Citation Impact. The systematic review proposing the recommendations for Oral Immunotherapy in CMA [8] has been cited 103 times with a 6.12 Field-Weighted Citation Impact. Thus, DRACMA influenced heavily the subsequent literature on CMA.
DRACMA publications
After ARIA (Allergic Rhinitis and its Impact on Asthma), DRACMA was the second guideline in allergy medicine focused on important patient outcomes, explicitly taking into consideration the patient’s values and preferences. It pioneered in applying a systematic approach to collecting the evidence, to separate the concepts of quality of evidence and strength of recommendations, and to transparently report the decision process. The method used for this CMA guideline was highlighted as an example of application of the GRADE methodology in an article cited 58 times [9]. The application of such principles to the diagnostic tests for CMA warranted a specific report, which has been cited 42 times [10].
Other articles reported on the global burden of CMA [11], and on its clinical aspects after the publication of the guideline [12,13,14].
Guidelines on diagnosis and treatment of food allergy before and after DRACMA
Prior to DRACMA, a handful of guideline documents for food allergy diagnosis and treatment had been issued by the main scientific societies in America and Europe [15,16,17]. National position papers and guidelines were available in the Netherlands [18], Finland [19], Spain [20], France [21], Germany [22] and Japan [23]. In general, these guidelines were intended for specific countries and/or for specific geographical areas, so they took stock of local factors of epidemiological, economic, organizational, and social nature. None of these documents used the GRADE methodology.
After 2010, other guidelines in the field of food allergy were proposed. One of them made use of the GRADE methodology in a way similar to DRACMA [24], another used some form of GRADE [25], and others were consensus-based documents [26,27,28,29,30]. Some national guidelines were also updated or issued [31,32,33,34]. During its 7 years, DRACMA was compared to other food allergy guidelines, illustrating how the values and preferences expressed by the writing committees can modify the recommendations [35, 36].
The number of citations may reflect the relevance of the different food allergy guidelines: the most cited is the National Institute of Allergy and Infectious Diseases (NIAID) guideline [24] (392 citations, 5.17 Field-Weighted Citation Impact). DRACMA stands second (241, 6.26), followed by the European Academy of Allergy and Clinical Immunology (EAACI) guidelines [25] (210, 18.68 Field-Weighted Citation Impact).
Guidelines on diagnosis and treatment of CMA before and after DRACMA
By 2010, a few consensus documents provided guidance on the diagnostic and therapeutic aspects of CMA in children [37, 38]. National position papers and guidelines had been produced in Germany [39, 40], Italy [41] and Argentina [42], reflecting general and local needs and vision.
After the publication, 93 WAO-affiliated national Allergy Societies endorsed the DRACMA guideline. Many of the national meetings of these societies hosted lectures on the topic. DRACMA was presented in many countries, in US, France, Italy, Brazil, Chile, Argentina, Kenya, Egypt, Thailand, and Indonesia, to name a few. In addition, some Allergy Societies outside of WAO, e.g. the Iranian, invited WAO lecturers to present on DRACMA. Following the DRACMA explicit invitations to national implementation, some scientific and regulatory bodies did discuss and actualize it in France [43, 44], United Kingdom [45, 46], Middle East [47], South Africa [29, 30]. In Mexico, the DRACMA recommendations were incorporated in a large specific guideline [48].
In other cases, the DRACMA guidelines were directly translated into the national languages, to overcome language barriers. This happened in Italy [49], in South America with the Spanish translation [50] and in China [51]. The Mandarin translation was also discussed to be actualized in the Chinese context [52].
After these discussions in many countries, DRACMA is now the most cited CMA guideline, followed by the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) guideline on cow’s-milk protein allergy [53] (179 citations, 16.56 Field-Weighted Citation Impact) and by the Italian CMA guideline [41] (51 citations, 4.02 Field-Weighted Citation Impact). The British Society of Allergy and Clinical Immunology (BSACI) guidelines [46] score 4th (42 citations, 11.57 Field-Weighted Citation Impact).
Economic consequences of DRACMA: Diagnosis
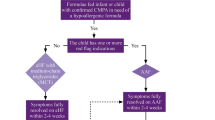
Among the diagnostic approaches proposed during the phases of national adaptation, the British example is of particular interest. In DRACMA, metanalyses of the available literature allowed us to calculate the performance characteristics of common diagnostic methods (skin prick test [SPT] and specific IgE determination, at the cut-off values of 3 mm wheal diameter and 0.35 kUA/L respectively) vs. the Oral Food Challenge (OFC) reference test. Assessing the clinical history, physicians can determine the diagnostic likelihoods estimating the pre-test probability of CMA. As examples, the pre-test probability will be low in cases of atopic dermatitis or Gastroesophageal Reflux Disease (GERD), average in case of immediate reactions or high in case of anaphylaxis. The DRACMA guidelines recommend – when possible – OFC for diagnosing CMA, to avoid the risk of anaphylactic reactions at home in SPT or sIgE false negative cases, unnecessary treatment for false positive cases and inappropriate resource utilization. However, some reasons (availability of appropriate staff, organizational obstacles, resource availability, etc.) may make it difficult to perform an OFC. In settings where OFC is not considered possible or opportune, a pre-test probability estimate may help physicians to reach a highly probable diagnosis using simpler diagnostic tests such as SPTs and/or specific IgE determination. These diagnostic pathways however, allow a small chance of false positive or negative results (Figs. 1 and 2) [13].
In settings where OFC is not considered a requirement, should in vitro specific IgE determination be used for the diagnosis of CMA in patients suspected of CMA and a positive result of a skin prick test? [13]
In settings where OFC is not considered a requirement, should in vitro specific IgE determination be used for the diagnosis of CMA in patients suspected of CMA and a negative result of a skin prick test? [13]
The cost of challenge test is reasonable in the majority of cases. In the British context however, challenges were considered "time-consuming and expensive" [46]. For this reason, the BSACI guidelines indicated UK as a setting where OFC is not considered an absolute requirement for the diagnosis of CMA. Taking stock of the DRACMA assessment of the probability of false-positive and false-negative diagnosis in case of high- medium- and low- pretest probability, they recommended the use of history (“typical” vs “non-typical”) and SPT as rule-out and diagnostic tests in clinical practice at the primary level. Especially for non IgE-mediated CMA, they underline the role of dietary elimination for the diagnosis. This approach, limiting the role of milk challenge to most doubtful cases, is similar to that proposed by the ESPGHAN "practical" guideline, issued in 2012 [53]. This choice is perhaps cost-effective, but may expose patients to the risk of overdiagnosis. As an example, in the Northern-Irish experience, the application of such strategy resulted in a reduction of prescriptions for symptomatic drugs for GERD, but in a steady increase in prescriptions for special formulas [54]. Although one may surmise that the diagnostic costs are reduced, the net costs for CMA treatment increased in that community [55]. This example illustrates how the application of a guideline can influence real life practices and economics.
Economic consequences of DRACMA: Treatment
The DRACMA recommendations proposed an appropriate substitute for different clinical situations. The question on substitute formulas was the following: "Should amino acid formula, extensively hydrolyzed whey or casein formula, soy formula or rice formula be used in children with IgE-mediated CMA?".
The answer to this clinical question was structured through the recommendations in the box.
Box: DRACMA recommendations for CMA management
Recommendation 7.1
In children with IgE-mediated CMA at high risk of anaphylactic reactions (prior history of anaphylaxis and currently not using extensively hydrolyzed milk formula), we suggest amino acid formula rather than extensively hydrolyzed milk formula (conditional recommendation/very low quality evidence).
Underlying values and preferences
This recommendation places a relatively high value on avoiding possible anaphylactic reactions and a lower value on avoiding the direct cost of amino acid formula in settings where the cost of amino acid formulas is high.
Remarks
In controlled settings, a trial feeding with an extensively hydrolyzed milk formula may be appropriate.
Recommendation 7.2
In children with IgE-mediated CMA at low risk of anaphylactic reactions, (no prior history of anaphylaxis or currently on extensively hydrolyzed milk formula), we suggest extensively hydrolyzed milk formula over amino acid formula (conditional recommendation/very-low quality evidence).
Underlying values and preferences
This recommendation places a relatively high value on avoiding the direct cost of amino acid formula in settings where the cost of amino acid formula is high. In settings where the cost of amino acid formula is lower, the use of amino acid formula may be equally reasonable.
Remarks
Extensively hydrolyzed milk formula should be tested in clinical studies before being used. If a new formula is introduced, one should carefully monitor if any adverse reactions develop after first administration.
In structuring these recommendations, formulas were rated according to a series of parameters. Among them, the price was explicitly indicated as an important factor. The DRACMA panel did a preliminary survey of the mean cost of different types of formulas worldwide (Table 1), from which it was found that feeding an infant with an extensively hydrolyzed formula (eHF) was 2.5 times less expensive than using an amino acid-based formula (AAF). Thus, even if the safety of AAF was higher than eHF, the latter was indicated as the first choice in CMA, except in cases of severe forms CMA with high reactivity (anaphylaxis or eosinophilic esophagitis), where AAF was recommended. Soy formulas (SF) were considered less useful to avoid reactions to soy unless they were more available and negative to skin testing. Extensively hydrolyzed rice formula (eHRF) is probably safer than eHFs, but it was considered at a lower level because it is not present in many countries (including UK).
As every recommendation reports the outcome that was considered most relevant by the expert panel (Box 1), they are flexible and can be subject to different interpretations when the importance of the outcomes in a particular country, or for a particular patient, is different. As the cost of the same formula differs substantially from country to country [56], the implementation of the recommendations may differ.
In recommendations 7.1 and 7.2, for example, cost makes AAF a second choice when the clinical risk is lower (see “values and preferences”). Elaborating on these considerations, an Italian company decided in 2012 to decrease the cost of their AAF by 30%, so that the cost of AAF dropped from 2.4 to 2 times that of eHF. This did modify the balance of recommendations for a substitute formula. AAF were proposed to children with even less severe forms of CMA, such as CM protein-induced atopic dermatitis.
This example illustrates how DRACMA guidelines did influence the formula market, making appropriate treatments affordable to larger layers of population. Naturally, this is only one of the factors for an appropriate care. In some countries, patients are reimbursed for AAF if “allergy” to eHF has been demonstrated, in others there are no reimbursement policies. This can expose to over-or under-use of special formulas.
Conclusions
DRACMA promoted a cultural debate among researchers and clinicians, improving the quality of diagnosis and clinical care. The accruing evidence on diagnosis and treatment supports the need for an update. Ideally, the new DRACMA guidelines should include non IgE-mediated CMA, particularly mild-moderate forms of CMA and chronic FPIES, as this part of the discipline has never been subjected to the strictest criteria for EBM, using the GRADE approach. We envisage the updated DRACMA will answer more clinical questions, serving the patients’ and the pediatricians’ needs in the various contexts.
References
Jensen-Jarolim E, Fiocchi A. World allergy organization journal: the editors look back at 2016. World Allergy Organ J. 2017;10:9.
Štulc T, Lánská V, Šnejdrlová M, Vrablík M, Prusíková M, Češka R. A comprehensive guidelines-based approach reduces cardiovascular risk in everyday practice: the VARO study. Arch Med Sci. 2017;13:705–10.
Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–30.
Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust. 2004;180(6 Suppl):S57–60.
Fiocchi A, Brozek J, Schunemann HJ, Bahna SL, von Berg A, Beyer K, et al. World allergy organization (WAO) diagnosis and rationale for action against Cow’s milk allergy (DRACMA) guidelines. WAO J. 2010;3:57–61.
Fiocchi A, Brozek J, Schunemann HJ, Bahna SL, von Berg A, Beyer K, et al. World allergy organization (WAO) diagnosis and rationale for action against Cow’s milk allergy (DRACMA) guidelines. Pediatr Allergy Immunol. 2010;21(Suppl 21):1–125.
Fiocchi A, Schünemann HJ, Brozek J, Restani P, Beyer K, Troncone R, et al. Diagnosis and rationale for action against Cow's milk allergy (DRACMA): a summary report. J Allergy Clin Immunol. 2010;126:1119–28.
Brozek JL, Terracciano L, Hsu J, Kreis J, Compalati E, Santesso N, et al. Oral immunotherapy for IgE-mediated cow's milk allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2012;42:363–74.
Brozek JL, Akl EA, Compalati E, Kreis J, Terracciano L, Fiocchi A, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;66:588–95.
Hsu J, Brozek JL, Terracciano L, Kreis J, Compalati E, Stein AT, et al. Application of GRADE: making evidence-based recommendations about diagnostic tests in clinical practice guidelines. Implement Sci. 2011;6(1):62.
Sackesen C, Assa'Ad A, Baena-Cagnani C, Ebisawa M, Fiocchi A, Heine RG, et al. Cow’s milk allergy as a global challenge. Curr Opin Allergy Clini Immunol. 2011;11:243–8.
Terracciano L, Schünemann H, Brozek J, Agostoni C, Fiocchi A. How DRACMA changes clinical decision for the individual patient in CMA therapy. Curr Opin Allergy Clin Immunol. 2012;12:316–22.
Fiocchi A, Schunemann H, Terracciano L, Albarini M, Martelli A, Landi M, et al. DRACMA one year after: which changes have occurred in diagnosis and treatment of CMA in Italy? Ital J Pediatr. 2011;37:53.
Assa'ad A, Fiocchi A. Guidelines change the diagnostic process of cow milk food allergy: problem-based learning. Curr Opin Allergy Clin Immunol. 2012;12:564–9.
Chapman JA, Bernstein IL, Lee RE, Oppenheimer J, Nicklas RA, Portnoy JM, et al. Food allergy: a practice parameter. Annals Allergy Asthma Immunol. 2006;96(3 Suppl 2):S1–68.
Bruijnzeel-Koomen C, Ortolani C, Aas K, Bindslev-Jensen C, Björkstén B, Moneret-Vautrin D, Wüthrich B. Adverse reactions to food. European academy of Allergology and clinical immunology subcommittee. Allergy. 1995;50:623–35.
Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS, et al. Adverse reactions to food Committee of American Academy of allergy, Asthma & Immunology. Work group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123(6 Suppl):S365–83.
Kneepkens CMF, Van Drongelen KI, Aarsen C. Landelijke standard voedselallergie bij zuigelingen [National standard for food allergy in infants]. 5th ed. Den Haag: Voedingscentrum; 2005. p. 80.
Finnish Paediatric Society. Food allergy in children. Duodecim. 2004;120:1524–38.
García BE, Gamboa PM, Asturias JA, López-Hoyos M, Sanz ML, Caballero MT, et al. Clinical immunology committee; Spanish society of allergology and clinical immunology. Guidelines on the clinical usefulness of determination of specific immunoglobulin E to foods. J Investig Allergol Clin Immunol. 2009;19:423–32.
Rancé F, Deschildre A, Villard-Truc F, Gomez SA, Paty E, Santos C, et al. SFAIC and SP2A workgroup on OFC in children. Oral food challenge in children: an expert review. Eur Ann Allergy Clin Immunol. 2009;41:35–49.
Werfel T, Erdmann S, Fuchs T, Henzgen M, Kleine-Tebbe J, Lepp U, et al. Approach to suspected food allergy in atopic dermatitis. Guideline of the task force on food allergy of the German society of allergology and clinical immunology (DGAKI), the medical Association of German Allergologists (ADA) and the German Society of Pediatric Allergology (GPA). J Dtsch Dermatol Ges. 2009;7:265–71.
Mukoyama T, Nishima S, Arita M, Ito S, Urisu A, Ebisawa M, et al. Food allergy committee, Japanese society of pediatric allergy and clinical immunology. Guidelines for diagnosis and management of pediatric food allergy in Japan. Allergol Int. 2007;56:349–61.
Boyce JA. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58.
Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008–25.
Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. J Allergy Clin Immunol. 2012;129:906–20.
Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Practice Parameter Workgroup. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134:1016–25.
Nowak-Węgrzyn A, Chehade M, Groetch ME, Spergel JM, Wood RA, Allen K, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: executive summary-workgroup report of the adverse reactions to foods committee, American academy of allergy, asthma & immunology. J Allergy Clin Immunol. 2017;139:1111–26.
Levin ME, Gray CL, Goddard E, Karabus S, Kriel M, Lang A, Manjra AI, Risenga S, Terblanche A, Van der Spuy DA, for the South African Food Allergy Working Group (SAFAWG). South African food allergy consensus document 2014. S Afr Med J. 2015;105(1):62–5.
Lang A, Van der Spuy DA, Goddard E, Terblanche A, Kriel M, Gray CL, Karabus S, Manjra AI, Risenga SM, Levin ME, for the South African Food Allergy Working Group (SAFAWG). Elimination diets and dietary interventions for the management of food allergies. S Afr Med J. 2015;105(1):71–2.
Subspecialty Groups of Child Health Care, the Society of Pediatrics, Chinese Medical Association; Editorial Board of Chinese Journal of Pediatrics. Recommendations for the diagnosis and management of food allergy in infants and young children. Zhonghua Er Ke Za Zhi. 2011;49:344–8.
Mäkelä M, Jartti T, Kolho KL, Poikonen S, Remes S, Schwab U, et al. Update on current care guideline: food allergy (children). Duodecim. 2015;131:694–5.
Urisu A, Ebisawa M, Ito K, Aihara Y, Ito S, Mayumi M, et al. Japanese Guideline for Food Allergy 2014. Allergol Int. 2014;63:399–419.
Walsh J, O'Flynn N. Diagnosis and assessment of food allergy in children and young people in primary care and community settings: NICE clinical guideline. Br J Gen Pract. 2011;61:473–5.
Venter C, Arshad SH. Guideline fever: an overview of DRACMA, US NIAID and UK NICE guidelines. Curr Opin Allergy Clin Immunol. 2012;12:302–15.
Ruszczyński M, Horvath A, Dziechciarz P, Szajewska H. Cow’s milk allergy guidelines: a quality appraisal with the AGREE II instrument. Clin Exp Allergy. 2016;46:1236–41.
Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, Staiano A, Dupont C. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Arch Dis Child. 2007;92:902–8.
Kemp AS, Hill DJ, Allen KJ, Anderson K, Davidson GP, Day AS, Heine RG, Peake JE, Prescott SL, Shugg AW, Sinn JK. Australian consensus panel. Guidelines for the use of infant formulas to treat cow’s milk protein allergy: an Australian consensus panel opinion. Med J Aust. 2008;188:109–12.
Niggemann B, Friedrichs F, Koletzko B, et al. Positionspapier. Das Vorgehen bei Sa¨uglingen mit Verdacht auf Kuhmilchproteinallergie. Padiatrische Allergologie. 2005;4:14–8.
Kirchlechner V, Dehlink E, Szepfalusi Z. Cow’s milk allergy: guidelines for the diagnostic evaluation. Klin Padiatr. 2007;219:201–5.
Caffarelli C, Baldi F, Bendandi B, Calzone L, Marani M, Pasquinelli P, Emilian Working Group on Pediatric Allergy and Gastroenterology. Cow’s milk protein allergy in children: a practical guide. Ital J Pediatr. 2010;36:5.
Orsia M, Fernández A, Follett FR, Marchisone S, Saiege G, Busonia VB, Tabacco O, Toca C. Alergia a la proteína de la leche de vaca. Propuesta de Guía para el manejo de los niños con alergia a la proteína de la leche de vaca. Arch Argent Pediatr. 2009;107:459–70.
Dupont C, Chouraqui JP, de Boissieu D, Bocquet A, Bresson JL, Briend A, et al. Comité de nutrition de la Société française de pédiatrie. Dietetic treatment of cow's milk protein allergy. Arch Pediatr. 2011;18:79–94.
Rancé F, Bidat E, Deschidre A. Les signes cliniques, le diagnostic et la prise en charge de l’allergie aux protéines du lait de vache d’après les recommandations internationals du DRACMA. Rev Francaise d’Allergologie. 2011;51:506–11.
Venter C, Brown T, Shah N, Walsh J, Fox AT. Diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy – a UK primary care practical guide. Clin Transl Allergy. 2013;3:23.
Luyt D, Ball H, Makwana N, et al. British Society for Allergy and Clinical Immunology (BSACI) guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy. 2014;44:642–72.
Vandenplas Y, Abuabat A, Al-Hammadi S, et al. Middle East consensus statement on the prevention, diagnosis, and management of cow’s milk protein allergy. Pediatr Gastroenterol Hepatol Nutr. 2014;17:61–73.
Montijo-Barrios E, López-Ugalde MV, Ramírez-Mayans J, Anaya-Flórez MS, Arredondo-García JL, Azevedo-Tenorio I, et al. Guía latinoamericana para el diagnóstico y tratamiento de alergia a las proteínas de la leche de vaca (GL-APLV). Rev Investig Clin. 2014;66(Suppl 2):S9–S72.
Fiocchi A, Brozek J, Schunemann HJ, Bahna SL, von Berg A, Beyer K, et al. Organizzazione mondiale dell’allergia: le line-guida DRACMA (diagnosi e terapia della allergia alle proteine del latte vaccino). Rivista di Immunologia ed Allergologia Pediatrica. 2011;26(S1):1–104.
Fiocchi A, Brozek J, Schunemann HJ, Bahna SL, von Berg A, Beyer K, et al. Pautas de la Organización Mundial sobre Alergia para el Diagnóstico y Fundamento de la Acción Contra la Alergia a la Leche de Vaca. http://www.alergia.org.ar/uploadalergia/dracma_wao.pdf. Accessed 16 Aug 2017.
Fiocchi A, Brozek J, Schunemann HJ, Bahna SL, von Berg A, Beyer K, et al. World allergy organization. The outline of world allergy organization (WAO) diagnosis and rationale for action against Cow's milk allergy (DRACMA) guidelines. Zhonghua Er Ke Za Zhi. 2012;50:510–5.
Li HQ. Intensive reading of world allergy organization (WAO) diagnosis and rationale for action against Cow's milk allergy (DRACMA) guideline. Zhonghua Er Ke Za Zhi. 2012;50:516–8.
Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. European Society of Pediatric Gastroenterology, Hepatology, and nutrition. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55:221–9.
Wauters L, Brown T, Venter C, Dziubak R, Meyer R, Brogan B, et al. Cow’s milk allergy prescribing is influenced by regional and National Guidance. J Pediatr Gastroenterol Nutr. 2016;62:765–70.
Fiocchi A, Fierro V, La Marra F. Interpreting the results of guideline implementation: a long and winding road. J Pediatr Gastroenterol Nutr. 2016;62:665–6.
Vandenplas Y, Alarcon P, Fleischer D, Hernell O, Kolacek S, Laignelet H, Lönnerdal B, Raman R, Rigo J, Salvatore S, Shamir R, Staiano A, Szajewska H, Van Goudoever HJ, von Berg A, Lee WS. Should partial Hydrolysates be used as starter infant formula? A working group consensus. J Pediatr Gastroenterol Nutr. 2016;62(1):22–35.
Acknowledgements
Not applicable.
Funding
This review is unfounded.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
AF conceived of the review, participated in its design and coordination and helped to draft the manuscript. LD, LT, and AM were the authors of specific parts of the review. All the authors reviewed the manuscript, and lent their reflection and clinical experience. LD did the draft and helped in its finalization. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fiocchi, A., Schunemann, H., Ansotegui, I. et al. The global impact of the DRACMA guidelines cow’s milk allergy clinical practice. World Allergy Organ J 11, 2 (2018). https://doi.org/10.1186/s40413-017-0179-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40413-017-0179-7