Abstract
The maintenance of oxygen homeostasis in human tissues is mediated by several cellular adaptations in response to low-oxygen stress, called hypoxia. A decrease in tissue oxygen levels is initially counteracted by increasing local blood flow to overcome diminished oxygenation and avoid hypoxic stress. However, studies have shown that the physiological oxygen concentrations in several tissues are much lower than atmospheric (normoxic) conditions, and the oxygen supply is finely regulated in individual cell types. The gastrointestinal tract has been described to subsist in a state of physiologically low oxygen level and is thus depicted as a tissue in the state of constant low-grade inflammation. The intestinal epithelial cell layer plays a vital role in the immune response to inflammation and infections that occur within the intestinal tissue and is involved in many of the adaptation responses to hypoxic stress. This is especially relevant in the context of inflammatory disorders, such as inflammatory bowel disease (IBD). Therefore, this review aims to describe the intestinal epithelial cellular response to hypoxia and the consequences for host interactions with invading gastrointestinal bacterial pathogens.
Similar content being viewed by others
Physiological oxygen concentrations
Researchers have aptly described three terms that characterize the different oxygen levels to which cells are exposed: “normoxia” that denotes atmospheric oxygen content, “physioxia” that signifies biologically sufficient oxygen concentration in tissues, and “hypoxia” that represents oxygen concentrations less than normal, indicating an oxygen deficit [1]. Hypoxia has a substantial effect on several cellular processes and has been shown to influence the pathogenesis of several diseases including gastrointestinal disorders, tumors, and cardiovascular diseases [2]. Within the human body, the different tissues are supplied with varying concentrations of oxygen, depending on their specific metabolic demands. Therefore, a state of oxygen concentration that is physiologically lower than atmospheric does not necessarily indicate a deficit in oxygen supply or the existence of hypoxic stress [3]. The intestinal tissue, for instance, when measured with a multiwire platinum surface electrode, showed an average oxygen concentration of around 7 %, at the serosal side of the small bowel [1]. Moreover, the intestine faces daily fluctuations in perfusion, with higher blood and oxygen flow after food intake as compared to fasting levels [4]. Furthermore, due to its distinctive structure within the body, with the highly vascularized and oxygenated subepithelial mucosa on one side and the severely oxygen deficient lumen on the other side, the gastrointestinal (GI) tract is characterized by a steep oxygen gradient across the epithelial layer [4]. Non-invasive measurement of tissue oxygen partial pressure (pO2) by electron paramagnetic resonance (EPR) oximetry showed oxygen levels ranging from 8 % in the small intestinal wall to around 3 % at the villus tip and less than 2 % in the intestinal lumen [5]. A basic schematic of this gradient is displayed in Fig. 1. Considering these diverse oxygen levels and the daily fluctuations experienced in tissues, these conditions of physiological oxygenation or physiological hypoxia were termed physioxia [3]. In addition to studying the gastrointestinal environment and the host-pathogen interactions that occur in this tissue, it is important to find an adequate cell culture model that on the one hand closely mimics physiological relevant conditions and on the other hand facilitates experimental procedures. A recent in vitro study on Caco-2 cultures, using non-invasive optical sensing techniques, showed pO2 values of around 3 % in the differentiated cells grown in a traditional tissue culture incubator, somewhat mimicking the in vivo physiological oxygen conditions [6]. These data further underline the significance of examining the effects of hypoxic adaptation on host-pathogen interactions in both in vivo and in vitro settings.
Schematic of gut oxygen gradient. The intestine faces daily fluctuations in blood flow and a steep oxygen gradient is present, extending from the highly vascularized and oxygenated subepithelial mucosa (4–8 %), across the epithelial and mucous layer (2–4 %), and into the severely oxygen-deficient lumen (<2 %). Arterial blood oxygen content is approximated as 80–100 % while venal blood oxygen content is approximated as 20 % [1, 4, 5]
Hypoxia during infections and inflammation
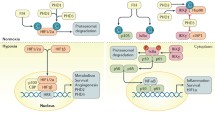
Hypoxic stress, that occurs when cellular oxygen demand is higher than its supply, is a commonplace in tissues faced with infection and inflammation [7, 8]. There are many factors that result in this oxygen deficit, including the demands of innate immune cells, such as neutrophils and macrophages that are recruited to the site of infection as well as those of invading pathogens that also consume oxygen [9, 10]. These increased oxygen demands, in addition to the requirements of the resident cells of the infected tissue, can cause a severe drop in available oxygen levels, resulting in a state of hypoxia. Infiltrating neutrophils play an important role in the host response to inflammation and result in depletion of oxygen, transcriptional changes, and aberrant vascularization [10]. Recruited polymorphonuclear neutrophils (PMNs) rapidly generate reactive oxygen species, mediated by a powerful oxidative burst, thus immensely increasing oxygen consumption. Activated PMNs elicit an almost 50-fold increase in oxygen demands and thus contribute to the hypoxic conditions in the inflamed tissue [7, 8].
A state of hypoxia resulting from infections has been shown in vivo in several studies. Infection of renal tubules with uropathogenic Escherichia coli (UPEC) in rats resulted in a severe drop in pO2 from 7 % in uninfected tissues to 5 % O2 1 h post-infection and finally to almost 0 % O2 within 4 h of infection, as measured by Clark-type microelectrodes [11]. This dramatic decrease in pO2 may be partially due to the recruitment of activated leukocytes that congest the capillaries and release reactive oxygen species as they translocate towards the site of infection [11]. Furthermore, tissues that subsist under conditions of chronic inflammation are shown to have a reduction in blood supply and a consequent loss of adequate oxygenation [9]. During inflammation and hypoxia, angiogenesis is induced to compensate for poor oxygenation; however, this may result in aberrant vasculature and contribute to the pathogenesis of chronic inflammation [7]. In IBD, vascular endothelial growth factor (VEGF)-dependent angiogenesis and increased production of vasoconstrictors were shown to result in abnormal microvasculature [8]. Figure 2 summarizes the events that lead to decreased oxygen levels in the intestinal tissue during an infection and chronic inflammation.
Intestinal tissue oxygen levels after infection or chronic inflammation. a One hour after infection, infiltration by neutrophils causes an increase in ROS production and subsequent decrease in oxygen levels, from 7 % to almost 5 %. Vasodilators are released to promote microvessel perfusion. Epithelial barrier is intact, and bacterial spread is contained. b As the infection progresses, pro-inflammatory cytokines are released and more PMNs are recruited to the tissue further decreasing local oxygen levels to less than 1 %. The epithelial layer is disrupted, and blood vessels become constricted because of clotting. Several hypoxia-dependent genes are upregulated. c In tissues with chronic inflammation, infiltrating neutrophils also lead to depletion of oxygen. Transcriptional changes in hypoxia-dependent genes along with aberrant vascularization create a severe hypoxic environment (2–4 %) [7, 8, 11]
The human gut is host to a large number of commensal bacteria that inhabit the lumen and epithelial mucosa of the lower intestine and has developed productive relationships with its microbiota; however, it remains highly vigilant against invading pathogens [12]. In cases when the balance of the normal flora is upset, or if the intestinal barrier is breached, infection can occur from invading pathogens or from overgrowth of endogenous pathogens [12]. Indeed, invasive enteropathogenic bacteria, including Salmonella, Shigella, and Yersinia, cause considerable damage to the mucosal layer and the intestinal epithelial cells as well as the lamina propria [12]. Enterocolitis, as the most common presentation of Yersinia enterocolitica infection, occurs primarily in young children, with a mean age of 24 months. The incubation period is 4–6 days, typically with a range of 1–14 days. Most cases are self-limited. However, concomitant bacteremia may occur in 20–30 % of infants younger than 3 months [13]. Besides the physical and structural damage that occur during those intestinal infections, many of the intestinal pathogens induce the expression of inflammatory and chemoattractive cytokines that collectively raise an immune response [14]. Therefore, it is a point of interest to investigate the fate of the epithelial layer of the intestinal tissue during a bacterial infection under hypoxia and whether the cellular adaptation mechanisms offer the host any protective or defensive advantages. Understanding the protective host response under hypoxia might finally help to develop new prophylactic or therapeutic strategies that might be supportive for the host during cellular stress response.
Transcriptional response to hypoxia
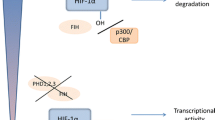
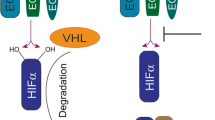
Living organisms have developed rather efficient mechanisms to maintain cellular homeostasis and to circumvent stressful conditions. At the cellular level, many genes are involved in the adaptation processes either in a regulatory capacity or in a functional manner. One very well-characterized regulator of the cellular response to low oxygen levels is the transcription factor hypoxia inducible factor 1 (HIF-1). HIF-1 has been found to bind to and induce the expression of several genes whose products promote erythropoiesis and angiogenesis and are involved in glucose transport and metabolism, thus initiating the cellular adaptation response to hypoxic stress [15, 16]. HIF-1 is a transcription factor consisting of two subunits: the oxygen regulated alpha (α) and a constitutively expressed beta (β) subunit, also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) [17]. HIF-1α protein is a global regulator of the energy homeostasis and cellular adaptation to hypoxia, and its stability is tightly regulated by the cellular oxygen concentration [18]. During conditions of adequate oxygenation, or normoxia, HIF-1α is rapidly degraded by binding of the von Hippel-Lindau tumor suppressor protein (pVHL) that subsequently targets it for ubiquitination and proteosomal degradation [19]. This process is mediated by oxygen- and iron-dependent prolyl hydroxylases (PHDs) that transfer a hydroxyl group onto two proline residues (P402 and P564) allowing for binding to pVHL [19]. Under hypoxic conditions, HIF-1α rapidly accumulates due to the interruption of its degradation pathway by inhibition of the oxygen-dependent hydroxylation [20].
Several HIF-1 target genes have been shown to mediate a protective effect on the mucosal layer through the upregulation of CD55, ecto-50 nucleotidase, MUC-3, intestinal trefoil factor, and P-glycoprotein [21]. This enhanced expression of barrier-protective genes may present an obstacle to invasive gastrointestinal bacteria that seek to invade by paracellular translocation across the epithelial layer, like Vibrio cholera and Clostridium difficile [22]. Furthermore, nuclear factor-kappa B (NF-κB), a central regulator of innate immunity and inflammatory processes, is activated in hypoxia, both in vitro and in vivo [23]. A complex connection exists between HIF-1 and NF-κB; both transcription factors have several target genes in common, and NF-κB activation can stabilize HIF-1α, yet HIF-1 can repress NF-κB activity during inflammation [21]. However, studies have also implicated HIF-independent NF-kB-mediated pathways in the host response at sites of inflammation, where NF-kB is required for the maintenance of epithelial barrier integrity [8, 21]. Conditional deletion of NF-kB in intestinal epithelial cells in mice led to an increased susceptibility to colitis in a murine model [24]. It has therefore been suggested that hypoxia regulates inflammatory responses through the activation of NFκB signaling pathway in a multi-factorial process [25]. This hypoxia-mediated induction of inflammatory responses may provide a better defense against invading pathogens.
Cellular adaptation to hypoxia
Bacterial invasion mechanisms: trigger or zipper
The intestinal epithelial layer is a crucial barrier against invading pathogens and resistance to infection; however, it is also the point of entry for many bacteria [22]. Since epithelial cells are non-phagocytic in nature, pathogens need to actively induce their own entry into host cells [26]. Thus, two major pathogen-mediated entry mechanisms have been identified: the binding of bacterial adhesins to host cell receptors (zipper mechanism) and the injection of bacterial effector proteins into the host cell cytosol (trigger mechanism), both of which are followed by cell signaling pathways that result in cytoskeletal remodeling and subsequent engulfment [26]. Among the common gastrointestinal pathogens, Y. enterocolitica utilizes the zipper mechanism while Shigella flexneri has been known to employ the trigger mechanism to invade host cells [27]. Pseudomonas aeruginosa, a generally opportunistic bacterium associated with nosocomial infections, has been increasingly found in the gastrointestinal tract of hospitalized patients [28]. P. aeruginosa internalizes into host epithelial cells by the trigger mechanism, using one of its injected effector proteins, ExoS that binds to the mammalian factor FXYD3 [29]. Interestingly, these abovementioned three intestinal pathogenic bacteria exhibit decreased levels of internalization into intestinal epithelial cells (for Y. enterocolitica and S. flexneri) or pulmonary epithelial cells (for P. aeruginosa) pre-incubated under 1 % O2 for 24 h [30, 31]. In light of these results, understanding the cellular effects of hypoxia on epithelial cells, e.g., membrane alterations, cytoskeletal rearrangements, or endoplasmatic reticulum (ER) stress during infections, offers a new potential to find targets for pharmacological interference. Thus, we sought to correlate this phenomenon of decreased internalization with the various hypoxia-induced cellular adaptations that have been published so far. However, we have to highlight the fact that two other well-known pathogens, Listeria monocytogenes and Salmonella typhimurium that internalize by the zipper and trigger mechanisms, respectively, exhibited increased entry into HT-29 enterocyte cells when cells are pre-incubated under 10 and 5 % hypoxia for 21 days [32]. The specific mode of entry of gastrointestinal pathogens, their effector proteins, and host receptors or targets as well as the proposed mechanism for hypoxia-mediated changes in internalization is presented in Table 1.
Membrane alterations
One region of the plasma membrane that poses great interest to host pathogen interactions is lipid rafts. Lipid rafts are ordered liquid domains rich in sphingolipids and cholesterol and segregated from less-ordered liquid domains composed of mainly unsaturated phospholipids [33]. Cell signaling, intracellular membrane transport, cell adhesion, and host-pathogen interactions are among the cell processes regulated by lipid rafts [34]. Therefore, any chemical and physical perturbations of plasma membrane structure or composition may have a dramatic effect on cellular processes that are associated with lipid rafts. In fact, hypoxic exposure leads to the selective remodeling of membrane lipids and proteins, more specifically to an increase in saturated fatty acid content due to the inability to perform β-oxidation in oxygen-limited conditions while amounts of phospholipids and free cholesterol remain unchanged [35]. In alveolar cells, mild hypoxia results in a significant increase in the cholesterol to phospholipids ratio causing a decrease in membrane fluidity, with no significant increase in lipid peroxidation [36]. This selective lipid enrichment and decrease in membrane fluidity under hypoxia is suggested as an adaptation response to regulate the function of membrane-bound proteins and their localization by decreasing endocytosis [35]. Furthermore, when membrane composition is altered, membrane-associated proteins are also most likely affected. The protein marker of caveolae, caveolin 1 (Cav-1), reveals reduced levels in the lipid microdomains while the total content of this protein the membranes remains unchanged, thus indicating a redistribution within the membrane [36]. These studies were performed in various types of cells, however, and not much is known about membrane alterations in intestinal epithelial cells. Considering that the main point of contact between pathogens and intestinal host cells is at the plasma membrane, it is possible that alterations in membrane-associated receptors may protect against bacterial invasion. Host β1 integrins, that are the main receptors for Y. enterocolitica, are lipid raft-associated proteins that require these platforms for clustering as well as recycling [37, 38]. In the absence of anchored and clustered receptors at the cell surface, due to hypoxia-mediated alterations, bacterial attachment and internalization may be greatly hindered, thus providing the host cells with protection against invading pathogens. In fact, we have shown a significant reduction in brush border membrane enrichment of β1 integrins under hypoxia, thus severely reducing Y. enterocolitica internalization into Caco-2 cells [39]. Furthermore, studies have shown that after 5-h incubation under 1 % O2, phosphatidylinositol activity is increased in hepatoma cells [40]. Since phosphatidylinositol is used by S. typhimurium to internalize into host cells, its accumulation under hypoxia may explain the increased pathogen entry [32]. On the other hand, S. flexneri effectors deplete phosphatidylinositol from the plasma membrane in order to limit membrane cytoskeletal interactions and facilitate entry into host cells [41]. An increase in membrane phosphatidylinositol levels may be the reason why Shigella entry into host cells is hindered under hypoxia [30].
Cytoskeletal rearrangements
Another key aspect of the cellular response to hypoxia is cytoskeletal adaptation. Studies have reported hypoxia-induced disorganization of the cytoskeletal network by disrupting F-actin filaments and by excessive cleavage of α-spectrin, an apical protein, that binds to the actin cytoskeleton and sodium transport proteins [42]. Furthermore, hypoxia has been shown to have a distinct effect on epithelial cells by disrupting the actin cytoskeleton and tight junctions, by mislocalization of occludin and reduction of the zonula occludens 1 [42, 43]. Furthermore, hypoxia regulates the Rho guanosine triphosphatases (GTPases) that modulate the activity of actin-binding proteins, by inhibiting their isoprenylation and thus resulting in decreased actin polymerization and eventually in impaired endocytosis [35]. Since many invasive pathogens, such as Shigella and Yersinia species, hijack the host cytoskeletal system in order to internalize into their target cells, any alterations in the cytoskeletal activity and structure can hinder this internalization process [27]. Oxygen-dependent modifications of the host cytoskeleton also significantly affect the paracellular permeability, intracellular transport, and the general endocytic uptake of particles in alveolar epithelial cells [42]. Since some of these bacteria are internalized into the intestinal epithelial cells via some form of endocytosis or membrane invagination, fragments of host plasma membrane will form the pathogen-containing compartments. Therefore, alterations in the host endocytic process caused by oxygen deficiency would also affect these compartments and ultimately influence intracellular bacterial survival [44].
Endoplasmic reticulum stress
The endoplasmic reticulum (ER) is responsible for protein translocation, folding, post-translational modifications, and finally, protein delivery to their proper target sites [45]. Under stressful conditions such as inflammation or infection, ER homeostasis is disturbed, causing the accumulation of misfolded or unfolded proteins in the ER lumen and ultimately leading to the induction of the unfolded protein response (UPR) [45]. The UPR consists of several steps, including (1) lowered protein biosynthesis and translocation into the ER, (2) increased expression of chaperones to aid in protein folding, (3) degradation of unfolded proteins, and finally, (4) apoptosis that eventually leads to pathogenic phenotypes [45]. Hypoxia has been shown to induce ER stress and can lead to the initiation of UPR, which in turn increases the transcription of pro-angiogenic genes such as VEGF by enhancing HIF-1α activity [46].
A large number of proteins are glycosylated in the ER not only to ensure proper trafficking to the cell surface in general [47] but also to differentially sort proteins to either the apical or basolateral membrane in polarized epithelial cells [48]. When ER stress is induced under hypoxia, and protein processing is altered, this may result in faulty trafficking and mislocalization of proteins that would normally act as cell surface receptors. The β1 integrin host receptors are indeed heavily glycosylated [49], and any alterations in the processing of these proteins may limit their availability at the cells surface, ultimately preventing bacterial internalization into host epithelial cells. Interestingly, many post-translational modifications to β integrins have been described after exposure to hypoxia, including uncontrolled cleavage by the protease calpain resulting in severely reduced protein levels at the surface of renal epithelial cells [50]. Furthermore, hypoxia significantly alters β1 integrin activation by affecting its maturation, glycosylation, and localization, thus interfering with various functions of this receptor and disrupting downstream signaling cascades [51, 52]. It is therefore hypothesized that hypoxia-mediated modifications to β1 integrins may obstruct Y. enterocolitica internalization into intestinal epithelial cells, as displayed in the model in Fig. 3. Transcriptional changes as well as post-translational modifications may also affect the internalization of L. monocytogenes and P. aeruginosa. L. monocytogenes internalizes into host cells via InlA and InlB that target E-cadherin and Met, the hepatocyte growth factor receptor, respectively [53]. Both of these host receptors displayed increased expression under hypoxia, possibly resulting in increased L. monocytogenes entry into enterocytes [54, 55]. P. aeruginosa entry utilizes the host receptor FXYD3 that both regulates and co-localizes with sodium-potassium ATPases [29]. Under hypoxia, Na-K-ATPase expression is decreased [56] and FXYD3 was found to be downregulated in breast and lung epithelial tumors [57, 58], all of which may play a role in the decreased internalization of P. aeruginosa into epithelial cells [31].
Hypothesized cellular adaptation models. Under normoxia, β1 integrins are properly glycosylated in the ER, are subsequently trafficked to the cell surface, and associate with lipid rafts. This allows binding to Yersinia enterocolitica invasin and bacterial internalization into host intestinal epithelial cell. Under hypoxia, HIF-1/hypoxia-induced ER stress and membrane alterations result in improper glycosylation and mislocalization of β1 integrins, thus reducing binding to and internalization of Y. enterocolitica
Mucosal immunity
The first barrier that microorganisms encounter in the intestine is a thick mucosal layer overlying the epithelial cells that provides protection against invading pathogens and other chemical or mechanical threats, composed mainly of glycoproteins called mucins [59]. During infections, mucins are commonly associated with pathogen adherence, and both S. typhimurium and Y. enterocolitica virulent strains were shown to specifically bind to mucins in the intestinal tract [60, 61]. Mucin expression can be increased by various cues, such as exposure to microbes, pro-inflammatory cytokines, and resident macrophages, thus providing a link between innate mucosal immunity, and inflammatory responses [59]. Increased expression of Muc 2, a major intestinal mucin, was shown to protect against infectious colitis by limiting Citrobacter rodentium translocation across the intestinal epithelial layer in mice [62]. Interestingly, hypoxia increases the expression of Muc 1 and Muc 2 in a HIF-1α-dependent manner in renal and peritoneal tumors, respectively [63, 64]. Whether this hypoxia-mediated increase in mucins aids in the entrapment of invading pathogs or serves to strengthen the epithelial barrier remains unclear. Besides transcriptional regulation of mucins, alterations in mucin glycosylation have been shown to occur, thus affecting microbial adhesion and circumventing mucus degradation by microbes [59]. As we have discussed in this review, hypoxia-induced ER stress can lead to alterations in glycosylation patterns, suggesting a potential role for hypoxia in mucin response to infections and their role in pathogen clearance.
Relevance in the context of inflammatory bowel diseases
The GI tract has been described to be in a state of constant, low-grade inflammation associated with hypoxia, with intestinal epithelial cells playing a pivotal role in mucosal immunity in response to this inflammation and in maintaining homeostasis [8]. Chronic gastrointestinal inflammatory conditions, such as Crohn’s disease and ulcerative colitis, are characterized by exaggerated inflammatory responses to the luminal microbiome and are aggravated by the resulting epithelial barrier dysfunction [65].
Interestingly, HIF-1α was found to be highly expressed in epithelial cells from both Crohn’s disease and ulcerative colitis patients [66]. The role of HIF-1α and its activated pathways was revealed to be a protective one, with suggested mechanisms that include improved barrier protection and prevention of epithelial cell apoptosis [21, 67]. Furthermore, conditional knockout of HIF-1α expression in intestinal epithelial cells exacerbated barrier injury and resulted in more severe symptoms in a murine colitis model [54]. A similar aggravation in inflammatory damage was seen in C. difficile toxin-induced colitis in mice lacking intestinal epithelial HIF-1α [68]. In contrast, increasing HIF-1α by pharmacological inhibition of its degradation substantially reduced the extent of injury caused by inflammatory damage in these colitis models [67, 68].
In light of increasing antibiotic resistance, novel approaches to treatment of infections are needed, and methods for boosting the host defense are currently being explored [69]. Because of its implication in the hypoxia-induced modulation of the immune response, HIF-1α has been considered as a possible novel therapeutic target [70]. Pharmacological manipulation of HIF-1α significantly improved the ability of keratinocytes to fight against skin infections [71]. Treatment with a HIF-1α agonist boosts the innate immune response of the intestinal epithelium in a murine colitis model [72].
These data strongly suggest that HIF-1α is a promising candidate for use as a therapeutic agent for the treatment of several pathological conditions. The ability of HIF-1α to boost the innate immune cells is a very attractive feature that can be effective in the treatment of multi-drug resistant bacterial infections or in immunocompromised patients [69]. Since the pharmacological stabilization of HIF-1α results in the increase of a number of antimicrobial host agents, the chances for the development of bacterial resistance are greatly diminished [70].
Ultimately, it is important to emphasize the relevance of studying hypoxia and the pathways involved in the adaptation to oxygen stress in the context of gastrointestinal perturbations and the host cell endeavors to maintain homeostasis.
References
Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15:1239–1253. doi:10.1111/j.1582-4934.2011.01258.x
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408. doi:10.1016/j.cell.2012.01.021
Guzy RD, Schumacker PT (2006) Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91:807–819. doi:10.1113/expphysiol.2006.033506
Taylor CT, Colgan SP (2007) Hypoxia and gastrointestinal disease. J Mol Med 85:1295–1300. doi:10.1007/s00109-007-0277-z
Fisher EM, Khan M, Salisbury R, Kuppusamy P (2013) Noninvasive monitoring of small intestinal oxygen in a rat model of chronic mesenteric ischemia. Cell Biochem Biophys 67:451–9. doi:10.1007/s12013-013-9611-y
Zeitouni NE, Fandrey J, Naim HY, von Köckritz-Blickwede M (2015) Measuring oxygen levels in Caco-2 cultures. Hypoxia 3:53–66. doi:10.2147/HP.S85625
Glover LE, Colgan SP (2011) Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology 140:1748–1755. doi:10.1053/j.gastro.2011.01.056
Colgan SP, Curtis VF, Campbell EL (2013) The inflammatory tissue microenvironment in IBD. Inflamm Bowel Dis 19:2238–44. doi:10.1097/MIB.0b013e31828dcaaf
Colgan SP, Taylor CT (2010) Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7:281–7. doi:10.1038/nrgastro.2010.39
Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP (2014) Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40:66–77. doi:10.1016/j.immuni.2013.11.020
Melican K, Boekel J, Månsson LE, Sandoval RM, Tanner G a G a, Källskog Ö, Palm F, Molitoris B a, Richter-Dahlfors A (2008) Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell Microbiol 10:1987–1998. doi:10.1111/j.1462-5822.2008.01182.x
Gorbach SL (1996) Microbiology of the gastrointestinal tract
Bottone EJ, Gullans CR, Sierra MF (1987) Disease spectrum of Yersinia enterocolitica serogroup 0:3, the predominant cause of human infection in New York City. Contrib Microbiol Immunol 9:56–60
Neish AS (2002) The gut microflora and intestinal epithelial cells: a continuing dialogue. Microbes Infect 4:309–317. doi:10.1016/S1286-4579(02)01543-5
Wang GL, Semenza GL (1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A 90:4304–4308. doi:10.1073/pnas.90.9.4304
Greijer A, van der Groep P, Kemming D, Shvarts A, Semenza G, Meijer G, van de Wiel M, Belien J, van Diest P, van der Wall E (2005) Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J Pathol 206:291–304. doi:10.1002/path.1778
Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454. doi:10.1128/MCB.12.12.5447.Updated
Wang GL, Jiang BH, Rue E a, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92:5510–5514. doi:10.1073/pnas.92.12.5510
Lee J-W, Bae S-H, Jeong J-W, Kim S-H, Kim K-W (2004) Hypoxia-inducible factor (HIF-1) alpha: its protein stability and biological functions. Exp Mol Med 36:1–12. doi:10.1038/emm.2004.1
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275. doi:10.1038/20459
Biddlestone J, Bandarra D, Rocha S (2015) The role of hypoxia in inflammatory disease (review). Int J Mol Med 35:859–869. doi:10.3892/ijmm.2015.2079
Balkovetz DF, Katz J (2003) Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect 5:613–619. doi:10.1016/S1286-4579(03)00089-3
Koong a C, Chen EY, Giaccia a J (1994) Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res 54:1425–1430
Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost E a, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D (2007) Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature 446:552–556. doi:10.1038/nature05590
Taylor CT, Cummins EP (2009) The role of NF-κB in hypoxia-induced gene expression. Ann N Y Acad Sci 1177:178–184. doi:10.1111/j.1749-6632.2009.05024.x
Alonso A, Del Portillo FG (2004) Hijacking of eukaryotic functions by intracellular bacterial pathogens. Int Microbiol 7:181–191
Dos Reis RS, Horn F (2010) Enteropathogenic Escherichia coli, Samonella, Shigella and Yersinia: cellular aspects of host-bacteria interactions in enteric diseases. Gut Pathog 2:8. doi:10.1186/1757-4749-2-8
Stoodley BJ, Thom BT (1970) Observations on the intestinal carriage of Pseudomonas aeruginosa. J Med Microbiol 3:367–75. doi:10.1099/00222615-3-3-367
Okuda J, Hayashi N, Okamoto M, Sawada S, Minagawa S, Yano Y, Gotoh N (2010) Translocation of Pseudomonas aeruginosa from the intestinal tract is mediated by the binding of ExoS to an Na, K-ATPase regulator, FXYD3. Infect Immun 78:4511–22. doi:10.1128/IAI.00428-10
Lima CBC, Dos Santos SA, De Andrade Junior DR (2013) Hypoxic stress, hepatocytes and CACO-2 viability and susceptibility to Shigella flexneri invasion. Rev Inst Med Trop Sao Paulo 55:341–346. doi:10.1590/S0036-46652013000500008
Schaible B, McClean S, Selfridge A, Broquet A, Asehnoune K, Taylor CT, Schaffer K (2013) Hypoxia modulates infection of epithelial cells by pseudomonas aeruginosa. PLoS One 8:1–11. doi:10.1371/journal.pone.0056491
Wells CL, VandeWesterlo E, Jechorek RP, Erlandsen SL (1996) Effect of hypoxia on enterocyte endocytosis of enteric bacteria. Crit Care Med 24:985–91
Lindner R, Naim HY (2009) Domains in biological membranes. Exp Cell Res 315:2871–2878. doi:10.1016/j.yexcr.2009.07.020
Lafont F, van der Goot FG (2005) Bacterial invasion via lipid rafts. Cell Microbiol 7:613–620. doi:10.1111/j.1462-5822.2005.00515.x
Ledoux S, Runembert I, Koumanov K, Michel JB, Trugnan G, Friedlander G (2003) Hypoxia enhances Ecto-5′-nucleotidase activity and cell surface expression in endothelial cells: role of membrane lipids. Circ Res 92:848–855. doi:10.1161/01.RES.0000069022.95401.FE
Botto L, Beretta E, Bulbarelli A, Rivolta I, Lettiero B, Leone BE, Miserocchi G, Palestini P (2008) Hypoxia-induced modifications in plasma membranes and lipid microdomains in A549 cells and primary human alveolar cells. J Cell Biochem 105:503–513. doi:10.1002/jcb.21850
Isberg RR, Leong JM (1990) Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861–871. doi:10.1016/0092-8674(90)90099-Z
Deuretzbacher A, Czymmeck N, Reimer R, Trulzsch K, Gaus K, Hohenberg H, Heesemann J, Aepfelbacher M, Ruckdeschel K (2009) B1 Integrin-dependent engulfment of Yersinia enterocolitica by macrophages is coupled to the activation of autophagy and suppressed by type III protein secretion. J Immunol 183:5847–5860. doi:10.4049/jimmunol.0804242
Zeitouni NE, Dersch P, Naim HY, von Köckritz-Blickwede M (2016) Hypoxia decreases invasin-mediated Yersinia enterocolitica internalization into Caco-2 cells. PLoS One 11:e0146103. doi:10.1371/journal.pone.0146103
Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C (2003) Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem 278:31277–85. doi:10.1074/jbc.M300763200
Brumell JH, Grinstein S (2003) Role of lipid-mediated signal transduction in bacterial internalization. Cell Microbiol 5:287–297. doi:10.1046/j.1462-5822.2003.00273.x
Bouvry D, Planès C, Malbert-Colas L, Escabasse V, Clerici C (2006) Hypoxia-induced cytoskeleton disruption in alveolar epithelial cells. Am J Respir Cell Mol Biol 35:519–527. doi:10.1165/rcmb.2005-0478OC
Molitoris BA, Dahl R, Hosford M (1996) Cellular ATP depletion induces disruption of the spectrin cytoskeletal network. Am J Physiol Ren Physiol 271:F790–798
Takeuchi A (1967) Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol 50:109–136
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–29. doi:10.1038/nrm2199
Pereira ER, Frudd K, Awad W, Hendershot LM (2014) Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF). J Biol Chem 289:3352–3364. doi:10.1074/jbc.M113.507194
Lodish H, Berk A, Zipursky SL E Al, Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000) Protein glycosylation in the ER and golgi complex. Mol Cell Biol. doi:http://www.ncbi.nlm.nih.gov/books/NBK21744/. Accessed 10 Nov 2015
Jacob R, Naim HY (2001) Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol 11:1444–1450. doi:10.1016/S0960-9822(01)00446-8
Kim LT, Ishihara S, Lee CC, Akiyama SK, Yamada KM, Grinnell F (1992) Altered glycosylation and cell surface expression of beta 1 integrin receptors during keratinocyte activation. J Cell Sci 103(Pt 3):743–753
Aoyama K, Ozaki Y, Nakanishi T, Ogasawara MS, Ikuta K, Aoki K, Blomgren K, Suzumori K (2004) Cleavage of integrin by mu-calpain during hypoxia in human endometrial cells. Am J Reprod Immunol 52:362–369
Zuk A, Bonventre JV, Brown D, Matlin KS (1998) Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol 275:C711–C731
Rana MK, Srivastava J, Yang M, Chen CS, Barber DL (2015) Hypoxia increases the abundance but not the assembly of extracellular fibronectin during epithelial cell transdifferentiation. J Cell Sci 128:1083–1089. doi:10.1242/jcs.155036
Cossart P, Pizarro-Cerda J, Lecuit M (2003) Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol 13:23–31. doi:10.1016/S0962-8924(02)00006-5
Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH (2004) Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114:1098–1106. doi:10.1172/JCI200421086
Tacchini L, Dansi P, Matteucci E, Desiderio MA (2001) Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis 22:1363–1371. doi:10.1093/carcin/22.9.1363
Gusarova GA, Trejo HE, Dada LA, Briva A, Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M, Sznajder JI (2011) Hypoxia leads to Na, K-ATPase downregulation via Ca(2+) release-activated Ca(2+) channels and AMPK activation. Mol Cell Biol 31:3546–56. doi:10.1128/MCB.05114-11
Yamamoto H, Mukaisho K, Sugihara H, Hattori T, Asano S (2011) Down-regulation of FXYD3 is induced by transforming growth factor-β signaling via ZEB1/δEF1 in human mammary epithelial cells. Biol Pharm Bull 34:324–9. doi:10.1248/bpb.34.324
Okudela K, Yazawa T, Ishii J, Woo T, Mitsui H, Bunai T, Sakaeda M, Shimoyamada H, Sato H, Tajiri M, Ogawa N, Masuda M, Sugimura H, Kitamura H (2009) Down-regulation of FXYD3 expression in human lung cancers: its mechanism and potential role in carcinogenesis. Am J Pathol 175:2646–2656. doi:10.2353/ajpath.2009.080571
Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin M a (2008) Mucins in the mucosal barrier to infection. Mucosal Immunol 1:183–197. doi:10.1038/mi.2008.5
Vimal DB, Khullar M, Gupta S, Ganguly NK (2000) Intestinal mucins: the binding sites for Salmonella typhimurium. Mol Cell Biochem 204:107–117
Mantle M, Husar SD (1994) Binding of Yersinia enterocolitica to purified, native small intestinal mucins from rabbits and humans involves interactions with the mucin carbohydrate moiety. Infect Immun 62:1219–27
Bergstrom KSB, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA (2010) Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 6:e1000902. doi:10.1371/journal.ppat.1000902
Aubert S, Fauquette V, Hemon B, Lepoivre R, Briez N, Bernard D, Van Seuningen I, Leroy X, Perrais M (2009) MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res 69:5707–5715. doi:10.1158/0008-5472.CAN-08-4905
Dilly AK, Lee YJ, Zeh HJ, Guo ZS, Bartlett DL, Choudry HA (2016) Targeting hypoxia-mediated mucin 2 production as a therapeutic strategy for mucinous tumors. Transl Res 169:19–30.e1. doi:10.1016/j.trsl.2015.10.006
Cummins EP, Doherty G a, Taylor CT (2013) Hydroxylases as therapeutic targets in inflammatory bowel disease. Lab Investig 93:378–383. doi:10.1038/labinvest.2013.9
Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris a L, Koukourakis MI (2003) Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol 56:209–213
Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT (2008) The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134:156–165. doi:10.1053/j.gastro.2007.10.012
Hirota S a, Fines K, Ng J, Traboulsi D, Lee J, Ihara E, Li Y, Willmore WG, Chung D, Scully MM, Louie T, Medlicott S, Lejeune M, Chadee K, Armstrong G, Colgan SP, Muruve D a, MacDonald J a, Beck PL (2010) Hypoxia-inducible factor signaling provides protection in clostridium difficile-induced intestinal injury. Gastroenterology 139:259–269.e3. doi:10.1053/j.gastro.2010.03.045
Bhandari T, Nizet V (2014) Hypoxia-inducible factor (HIF) as a pharmacological target for prevention and treatment of infectious diseases. Infect Dis Ther 159–174. doi: 10.1007/s40121-014-0030-1
Zinkernagel AS, Johnson RS, Nizet V (2007) Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med 85:1339–1346. doi:10.1007/s00109-007-0282-2
Zinkernagel AS, Peyssonnaux C, Johnson RS, Nizet V (2008) Pharmacologic augmentation of hypoxia-inducible factor-1alpha with mimosine boosts the bactericidal capacity of phagocytes. J Infect Dis 197:214–217. doi:10.1086/524843
Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP (2008) Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134:145–155. doi:10.1053/j.gastro.2007.09.033
Tran Van Nhieu G, Bourdet-Sicard R, Dumenil G, Blocker A, Sansonetti PJ (2000) Bacterial signals and cell responses using Shigella entry into epithelial cells. Cell Microbiol 2:187–193. doi:10.1046/j.1462-5822.2000.00046.x
Acknowledgements
This work was partially supported by DFG grant KO 3552/4-1 (MvK-B); NZ was funded by the German Academic Exchange Service (DAAD), and SC was funded by the Mahanakorn University of Technology, Thailand.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NZ and SC collected the information and wrote the manuscript. MvKB and HYN critically revised the manuscript. All authors contributed to the drafting and revising of the paper and agreed to be accountable for all aspects of the work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zeitouni, N.E., Chotikatum, S., von Köckritz-Blickwede, M. et al. The impact of hypoxia on intestinal epithelial cell functions: consequences for invasion by bacterial pathogens. Mol Cell Pediatr 3, 14 (2016). https://doi.org/10.1186/s40348-016-0041-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40348-016-0041-y