Abstract
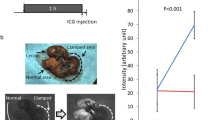
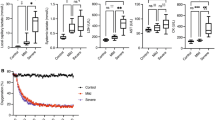
We noninvasively monitored the partial pressure of oxygen (pO2) in rat’s small intestine using a model of chronic mesenteric ischemia by electron paramagnetic resonance oximetry over a 7-day period. The particulate probe lithium octa-n-butoxynaphthalocyanine (LiNc-BuO) was embedded into the oxygen permeable material polydimethyl siloxane by cast-molding and polymerization (Oxy-Chip). A one-time surgical procedure was performed to place the Oxy-Chip on the outer wall of the small intestine (SI). The superior mesenteric artery (SMA) was banded to ~30 % of blood flow for experimental rats. Noninvasive measurement of pO2 was performed at the baseline for control rats or immediate post-banding and on days 1, 3, and 7. The SI pO2 for control rats remained stable over the 7-day period. The pO2 on day-7 was 54.5 ± 0.9 mmHg (mean ± SE). SMA-banded rats were significantly different from controls with a noted reduction in pO2 post banding with a progressive decline to a final pO2 of 20.9 ± 4.5 mmHg (mean ± SE; p = 0.02). All SMA-banded rats developed adhesions around the Oxy-Chip, yet remained asymptomatic. The hypoxia marker Hypoxyprobe™ was used to validate the low tissue pO2. Brown cytoplasmic staining was consistent with hypoxia. Mild brown staining was noted predominantly on the villus tips in control animals. SMA-banded rats had an extended region of hypoxic involvement in the villus with a higher intensity of cytoplasmic staining. Deep brown stainings of the enteric nervous system neurons and connective tissue both within layers and in the mesentery were noted. SMA-banded rats with lower pO2 values had a higher intensity of staining. Thus, monitoring SI pO2 using the probe Oxy-Chip provides a valid measure of tissue oxygenation. Tracking pO2 in conditions that produce chronic mesenteric ischemia will contribute to our understanding of intestinal tissue oxygenation and how changes impact symptom evolution and the trajectory of chronic disease.




Similar content being viewed by others
References
Kulkarni, A. C., Kuppusamy, P., & Parinandi, N. (2007). Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxidants & Redox Signaling, 9, 1717–1730.
Kutala, V. K., Khan, M., Angelos, M. G., & Kuppusamy, P. (2007). Role of oxygen in postischemic myocardial injury. Antioxidants & Redox Signaling, 9, 1193–1206.
Sandek, A., Bauditz, J., Swidsinski, A., Buhner, S., Weber-Eibel, J., von Haehling, S., et al. (2007). Altered intestinal function in patients with chronic heart failure. Journal of the American College of Cardiology, 50, 1561–1569.
Parks, D. A., Bulkley, G. B., Granger, D. N., Hamilton, S. R., & McCord, J. M. (1982). Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology, 82, 9–15.
Phillips, J. P., Kyriacou, P. A., Jones, D. P., Shelley, K. H., & Langford, R. M. (2008). Pulse oximetry and photoplethysmographic waveform analysis of the esophagus and bowel. Current Opinion in Anaesthesiology, 21, 779–783.
van Noord, D., Mensink, P. B., de Knegt, R. J., Ouwendijk, M., Francke, J., van Vuuren, A. J., et al. (2011). Serum markers and intestinal mucosal injury in chronic gastrointestinal ischemia. Digestive Diseases and Sciences, 56(2), 506–512.
Meenakshisundaram, G., Eteshola, E., Pandian, R. P., Bratasz, A., Selvendiran, K., Lee, S. C., et al. (2009). Oxygen sensitivity and biocompatibility of an implantable paramagnetic probe for repeated measurements of tissue oxygenation. Biomedical Microdevices, 11, 817–826.
Meenakshisundaram, G., Eteshola, E., Pandian, R. P., Bratasz, A., Lee, S. C., & Kuppusamy, P. (2009). Fabrication and physical evaluation of a polymer-encapsulated paramagnetic probe for biomedical oximetry. Biomedical Microdevices, 11, 773–782.
Eteshola, E., Pandian, R. P., Lee, S. C., & Kuppusamy, P. (2009). Polymer coating of paramagnetic particulates for in vivo oxygen-sensing applications. Biomedical Microdevices, 11, 379–387.
Khan, M., Kutala, V. K., Vikram, D. S., Wisel, S., Chacko, S. M., Kuppusamy, M. L., et al. (2007). Skeletal myoblasts transplanted in the ischemic myocardium enhance in situ oxygenation and recovery of contractile function. American Journal of Physiology, 293, H2129–H2139.
Khan, M., Kutala, V. K., Wisel, S., Chacko, S. M., Kuppusamy, M. L., Kwiatkowski, P., et al. (2008). Measurement of oxygenation at the site of stem cell therapy in a murine model of myocardial infarction. Advances in Experimental Medicine and Biology, 614, 45–52.
Pandian, R. P., Parinandi, N. L., Ilangovan, G., Zweier, J. L., & Kuppusamy, P. (2003). Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radical Biology Medicine, 35, 1138–1148.
Chacko, S. M., Khan, M., Kuppusamy, M. L., Pandian, R. P., Varadharaj, S., Selvendiran, K., et al. (2009). Myocardial oxygenation and functional recovery in infarct rat hearts transplanted with mesenchymal stem cells. American Journal Physiology Heart Circulation Physiology, 296, H1263–H1273.
Tarhan, O. R., Barut, I., Sutcu, R., Akdeniz, Y., & Akturk, O. (2006). Pentoxifylline, a methyl xanthine derivative, reduces peritoneal adhesions and increases peritoneal fibrinolysis in rats. Tohoku Journal of Experimental Medicine, 209, 249–255.
Wu, B., Qiu, W., Wang, P., Yu, H., Cheng, T., Zambetti, G. P., et al. (2007). p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut, 56, 645–654.
Granger, D. N., Hollwarth, M. E., & Parks, D. A. (1986). Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiology Scandinavia Supplement, 548, 47–63.
Mellstrom, A., Mansson, P., Jonsson, K., & Hartmann, M. (2009). Measurements of subcutaneous tissue pO2 reflect oxygen metabolism of the small intestinal mucosa during hemorrhage and resuscitation. An experimental study in pigs. European Surgical Research, 42, 122–129.
Yung, L. M., Leung, F. P., Yao, X., Chen, Z. Y., & Huang, Y. (2006). Reactive oxygen species in vascular wall. Cardiovascular & Hematological Disorders, 6, 1–19.
Cizova, H., Lojek, A., Kubala, L., & Ciz, M. (2004). The effect of intestinal ischemia duration on changes in plasma antioxidant defense status in rats. Physiological Research, 53, 523–531.
Meredith, I. T., Currie, K. E., Anderson, T. J., Roddy, M. A., Ganz, P., & Creager, M. A. (1996). Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. American Journal of Physiology, 270, H1435–H1440.
Loscalzo, J., & Vita, J. A. (1994). Ischemia, hyperemia, exercise, and nitric oxide. Complex physiology and complex molecular adaptations. Circulation, 90, 2556–2559.
Huang, A. L., Silver, A. E., Shvenke, E., Schopfer, D. W., Jahangir, E., Titas, M. A., et al. (2007). Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 2113–2119.
Goethals, L., Debucquoy, A., Perneel, C., Geboes, K., Ectors, N., De Schutter, H., et al. (2006). Hypoxia in human colorectal adenocarcinoma: comparison between extrinsic and potential intrinsic hypoxia markers. International Journal of Radiation Oncology Biology Physics, 65, 246–254.
Bohlen, H. G. (1998). Integration of intestinal structure, function, and microvascular regulation. Microcirculation, 5, 27–37.
Bohlen, H. G. (1980). Intestinal mucosal oxygenation influences absorptive hyperemia. American Journal of Physiology, 239, H489–H493.
Hall, P. A., Coates, P. J., Ansari, B., & Hopwood, D. (1994). Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. Journal of Cell Science, 107(Pt 12), 3569–3577.
Kerr, J. F., Wyllie, A. H., & Currie, A. R. (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer, 26, 239–257.
Potten, C. S., & Booth, C. (1997). The role of radiation-induced and spontaneous apoptosis in the homeostasis of the gastrointestinal epithelium: A brief review. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 118, 473–478.
Baudelet, C., & Gallez, B. (2004). Effect of anesthesia on the signal intensity in tumors using BOLD-MRI: comparison with flow measurements by Laser Doppler flowmetry and oxygen measurements by luminescence-based probes. Magnetic Resonance Imaging, 22, 905–912.
Holmdahl, L., & Risberg, B. (1997). Adhesions: Prevention and complications in general surgery. European Journal of Surgery, 163, 169–174.
Holmdahl, L., & Ivarsson, M. L. (1999). The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. European Journal of Surgery, 165, 1012–1019.
Brauner, A., Hylander, B., & Wretlind, B. (1993). Interleukin-6 and interleukin-8 in dialysate and serum from patients on continuous ambulatory peritoneal dialysis. American Journal of Kidney Disease, 22, 430–435.
Brauner, A., Hylander, B., & Wretlind, B. (1996). Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-1 receptor antagonist in dialysate and serum from patients on continuous ambulatory peritoneal dialysis. American Journal of Kidney Disease, 27, 402–408.
Saed, G. M., Zhang, W., & Diamond, M. P. (2000). Effect of hypoxia on stimulatory effect of TGF-beta 1 on MMP-2 and MMP-9 activities in mouse fibroblasts. Journal of the Society of Gynecological Investigation, 7, 348–354.
Saed, G. M., & Diamond, M. P. (2002). Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-beta1 in human peritoneal fibroblasts. Fertility and Sterility, 78, 144–147.
Milligan, D. W., & Raftery, A. T. (1974). Observations on the pathogenesis of peritoneal adhesions: A light and electron microscopical study. British Journal of Surgery, 61, 274–280.
Terada, L. S., Guidot, D. M., Leff, J. A., Willingham, I. R., Hanley, M. E., Piermattei, D., & Repine, J. E. (1992). Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proceedings of the National Academy of Science of the United States of America, 89, 3362–3366.
Snoj, M. (1993). Intra-abdominal adhesion formation is initiated by phospholipase A2. Medical Hypotheses, 41, 525–528.
Vikram, D. S., Bratasz, A., Ahmad, R., & Kuppusamy, P. (2007). A comparative evaluation of EPR and OxyLite oximetry using a random sampling of pO2 in a murine tumor. Radiation Research, 168, 308–315.
Acknowledgments
This study was supported by a Career Training Award (K01-NR009787-01) from the National Institute of Health: National Institute of Nursing Research to E.M. Fisher. We thank Dr. Sheau-Huey Chiu for assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fisher, E.M., Khan, M., Salisbury, R. et al. Noninvasive Monitoring of Small Intestinal Oxygen in a Rat Model of Chronic Mesenteric Ischemia. Cell Biochem Biophys 67, 451–459 (2013). https://doi.org/10.1007/s12013-013-9611-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9611-y