Abstract
Background
Cognitive changes associated with mild cognitive impairment or mild dementia can lead to difficulties in completing instrumental activities of daily living. The ability to live independently at home and in the community is often compromised due to the inability to complete these activities. Cognitive interventions have been reported as beneficial in maintaining or improving cognitive functions among this group of adults. However, the effectiveness of different types of cognitive interventions on the performance of instrumental activities of daily living in older adults with mild cognitive impairment and mild dementia is not well established. The aim of this paper is to develop a protocol for a systematic review and meta-analysis to investigate the effectiveness of cognitive interventions in maintaining or improving the performance of instrumental activities of daily living in individuals with mild cognitive impairment or mild dementia.
Methods
Randomised control studies which investigate the effectiveness of cognitive interventions on the performance in instrumental activities of daily living for older adults with mild cognitive impairment and mild dementia will be sought. A systematic search will be conducted in five databases: CINAHL, MEDLINE, EMBASE, PsycINFO and Cochrane Central Register of Controlled Trials. The search strategy was developed with assistance from a health science librarian. Two independent reviewers will perform the study selection and data extraction. Quality assessment will be implemented using the Physiotherapy Evidence Database (PEDro) scale. A narrative synthesis of the findings will be used to report outcomes of all included studies. If appropriate, a meta-analysis will combine the results of individual studies.
Discussion
This systematic review and meta-analysis will determine the effectiveness of cognitive interventions in maintaining or improving the performance of IADL in individuals with MCI or mild dementia. It is anticipated that the results will inform rehabilitation professionals of the most effective cognitive interventions to be implemented into clinical practice. It will potentially provide substantial benefit to both the persons with MCI or dementia and the health care system by keeping more people out of full-time care and allowing those in full-time care to require less intensive support.
Systematic review registration
PROSPERO CRD42016042364
Similar content being viewed by others
Background
Daily activities can be subcategorised as either basic or instrumental activities. Basic daily activities (BADL) are activities that are concerned with taking care of one’s own body and encompass 10 categories: bathing/showering, bowel and bladder management, dressing, eating, feeding, functional mobility, personal device care, personal hygiene and grooming, sexual activity and toilet hygiene [1]. Instrumental activities of daily living are more complex daily activities. They encompass 12 categories: care of others, care of pets, child-rearing, communication management, driving and community mobility, financial management, health management and maintenance, home establishment and management, meal preparation and clean-up, religious observance, safety and emergency maintenance, and shopping [1]. Engagement in these activities is required for people to participate in home and community life.
The ability to complete BADL usually remains intact among those with MCI or mild dementia [2, 3]. Impairment in BADL activities of daily has been shown to manifest after impairments of instrumental activities of daily living (IADL) [4]. There is a positive relationship between cognitive function and performance of IADL [5,6,7,8,9,10]. IADL demand more complex neuropsychological processing than BADL and are more susceptible to the subtle deterioration associated with cognitive decline [11]. It has been demonstrated that deterioration in memory and other cognitive functions may result in problems performing IADL, and thus, impact on people’s ability to independently live in the community [12,13,14]. For example, de Paula et al. [6] examined the subdomains of memory and executive functioning, finding that episodic memory and executive function correlated with IADL performance in domestic chores, telephone use, meal preparation and laundry (ps < .p5) as well as significantly predicted performance in financial management, shopping, medication management and using transportation (ps < .05). Similar results were found in Cahn-Weiner et al. [15] that memory and executive function were significantly associated with IADL performance (p = .001). Similarly, Farias et al. [16] also revealed that change in executive function was associated with a change in IADL performance (p < .001). Thus, a greater degree of decline in memory and executive function were associated with greater functional decline. A systematic review [17] examining IADL performance of individuals with MCI compared with cognitively healthy individuals and people with dementia found that people with MCI had intermediate functional performance in more complex tasks requiring higher cognitive demand such as telephone use, medication management and keeping appointments between cognitively healthy controls people with mild dementia, particularly.
As part of the normal ageing process, older adults may experience deterioration in cognition in which their cognitive functioning negatively affects their ability to perform IADL [18]. These cognitive changes appear to be greater in individuals with MCI and even more significant in individuals with dementia [17, 19, 20]. MCI and dementia are classified as neurocognitive disorders, whereby there is evidence of an acquired cognitive decline in one or more neurocognitive domains [21]. Deficits may be present in any of the six cognitive domains outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). These domains are complex attention, executive function, learning and memory, language, perceptual-motor function and social cognition. Cognitive interventions, which address the various cognitive domains, may be vital to maintaining or preventing a decline of cognitive function and subsequent IADL performance in individuals in the pre-clinical or early stages of dementia.
Cognitive interventions include (1) cognitive training, (2) cognitive rehabilitation and (3) cognitive stimulation [22]. These three intervention approaches feature in current literature relating to individuals with MCI and dementia as interventions targeting specific cognitive functions or interventions targeting functional performance in activities including IADL [22, 23].
Cognitive training consists of practising cognitive tasks, focussing on improving cognitive functions in areas such as memory, attention, problem solving and calculation. Cognitive training aims to improve cognitive functions in one or more cognitive domains [22, 24].
Unlike cognitive training, cognitive rehabilitation does not aim to improve cognitive functions specifically. Instead, the aim of cognitive rehabilitation is to address the problems in daily activity performance as a result of the decline in cognitive functions. Cognitive rehabilitation focuses on identifying goals to enhance daily activity performance, providing a tailored intervention for each person. Interventions often include providing compensatory and adaptive strategies and are targeted at improving performance in specific daily activities. Examples of cognitive rehabilitation include memory retrieval techniques, activity or environment modification and errorless learning [22, 25].
Cognitive stimulation is another intervention strategy that promotes engagement in activities to stimulate general cognitive and social functioning in a non-specific manner. Examples of cognitive stimulation include participating in group discussions, book clubs, quizzes and trivia, or music-related activities [24, 25].
A therapy program that utilises a combination of cognitive training, rehabilitation and/or stimulation with individuals with MCI or early stages of dementia may have the greatest benefit because they continue to retain the ability and the cognitive capacity associated with learning and applying new skills [26]. These three cognitive interventions are commonly adopted to assist people living with MCI or dementia as there is currently no proven curative therapy [23, 27]. Therefore, early cognitive intervention becomes vital in maintaining cognitive functions and daily activity performance in older adults.
Due to varying criteria for MCI and the difficulties with diagnosis, it is difficult to determine the prevalence of the disease [28,29,30]. A Canadian Health and Aging study reported an estimated population prevalence of 1.03 to 3.02% when adopting different definitions for MCI [29]. The range in prevalence between 3% and 19% was also demonstrated in a population-based epidemiological study [31]. Another review [32] reported prevalence estimates for MCI ranging from 16 to 20%.
Although the exact prevalence of MCI is difficult to determine, it has become widely recognised that the mild cognitive changes associated with MCI are a probable transitional period between normal ageing and a clinical diagnosis of dementia [33,34,35,36]. A study of 133 individuals with MCI and found a conversion rate from MCI to Alzheimer’s disease type dementia to be a staggering 30.5% [37]. According to the World Health Organization, around 50 million people are living with dementia. The total number of people with dementia is expected to increase to 82 million in 2030 and 152 million by 2050 [38]. In 2015, it was estimated that an equivalent value of 1.1% of global gross domestic product would be required to cover the total global societal cost of dementia which was estimated to be USD 818 billion [38]. Mild cognitive impairment, therefore, can constitute a substantial economic burden for public health systems.
With the increasing prevalence of cognitive impairment and dementia in the aging population [39,40,41,42], there is a need to review the efficacy of cognitive interventions to maintain or improve function. To date, reviews have reported the effectiveness of cognitive interventions on cognitive function, particularly on memory. Although individuals with MCI may have preserved functional abilities, it is well established that they may experience subtler difficulties such as making more errors or needing more time than healthy individuals to perform complex IADL. There has not been a systematic review examining the effectiveness of cognitive interventions directly on IADL across the continuum of cognitive decline from MCI to mild dementia. For example, systematic reviews by Simon et al. [25] and Jean et al. [43], both examined the impact of cognitive interventions for MCI on daily activity performance but only as a secondary outcome, and the results on specific IADL were not reported.
This current review will extend previous systematic reviews by including the effectiveness of cognitive interventions on the performance of IADL in all subtypes of MCI and mild dementia. The associated decline in cognition and decreased performance of IADL are not only associated with reduced independence and quality of life [7], but also have far-reaching economic implications and increased health care costs associated that may present challenges to the current health care system [7, 44]. For example, the total direct cost of dementia was $8799 million in Australia and is projected to increase two-fold in 20 years’ time [45]. Most of the direct cost goes to hospitalisation, attending general practitioners and specialists and providing care. Facilitating performance of IADL in people with MCI or mild dementia is an important strategy to aid successful community-based living and reduce the need for additional care and health services. This systematic review and meta-analysis will summarise the available evidence regarding the efficacy of cognitive interventions on the performance of IADL in adults with MCI or mild dementia and highlight areas for further research into cognitive interventions to promote IADL functioning for independent living.
Objectives
The objectives of this review are to identify, evaluate and synthesise all available randomised controlled trials of cognitive interventions (cognitive training, cognitive rehabilitation and cognitive stimulation) targeted at maintaining or improving IADL performance in older adults with MCI or mild dementia.
Methods
A systematic review utilising narrative synthesis and a meta-analytical approach will be conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [46]. The protocol of this systematic review has been registered in PROSPERO (registration number CRD42016042364).
Eligibility criteria
Types of studies
Studies included will report (1) cognitive interventions used with older adults with MCI or mild dementia and (2) performance in at least one IADL at baseline and post-treatment. Studies that are published in the English language in a peer-reviewed journal will be eligible. All randomised controlled trials with participants entering into both arms of the trial will be reported. Comparative studies with and without concurrent controls, such as non-randomised experimental trials, cohort studies, case-control studies, single-arm studies and case series, will be excluded from this review.
Types of participants
Studies with participants aged 60 or above, residing in either the community or within a residential aged care setting, and with a diagnosis of MCI or mild dementia as outlined by one of the following criteria, will be included:
-
World Health Organization’s International Classification of Diseases code [47];
-
National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria [48];
-
American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders [21];
-
Clinical Dementia Rating scale [49];
-
Blessed Dementia Rating Scale [50];
-
Petersen’s Diagnostic Criteria for MCI [51];
-
Mayo Clinic Diagnostic Criteria for MCI [35];
-
National Institute on Aging and the Alzheimer’s Association [52]; or
-
International Working Group on MCI Diagnostic Criteria [53].
Studies with the study sample under 60 years of age or with moderate to severe dementia will be excluded. Studies reporting participants with a diagnosis of MCI or mild dementia utilising an alternative diagnostic criterion will be considered if the diagnostic criteria are standardised, valid and reliable. Two reviewers (NT and KL) will review the available literature to reach a consensus as to whether studies utilising other diagnostic criteria will be included.
Types of interventions and comparisons
Cognitive interventions of interest are cognitive training, cognitive rehabilitation and cognitive stimulation. Delivery of these interventions is not limited to a specific mode. They can include face-to-face, computer-administered, individual or group interventions. Interventions delivered in any setting, inclusive of inpatient and outpatient hospital settings, community-based programs, rehabilitation settings, adult day support facilities and residential aged care facilities, will be included.
Interventions may be compared with active controls (for example, another rehabilitation intervention, such as an exercise program [54], caregiver training [55] or dietary changes [56]) or an inactive control group (for example, wait-list control or standard care). Studies which compare two cognitive interventions without control or standard care arm will be excluded. Interventions of any duration, frequency, intensity and delivery will be included.
Types of outcome measures
The outcome of interest is improved or maintained IADL performance. Studies must include at least one outcome measure assessing the performance of one or more IADL. The IADL will be included if it falls under one of the following 12 categories: care of others, care of pets, child-rearing, communication management, driving and community mobility, financial management, health management and maintenance, home establishment and management, meal preparation and clean-up, religious and spiritual activities and expression, safety and emergency maintenance, and shopping [1]. Outcomes can be performance-based assessed by therapists, self-reported by the participant or informant reported by a caregiver or significant other. Both standardised and non-standardised assessments will be included.
Information sources
Search strategy and study selection searches will be undertaken in OVID SP versions of MEDLINE and EMBASE, EBSCO versions of CINAHL and PsycINFO and the Cochrane Central Register of Controlled Trials.
In addition to the studies retrieved from the above databases, reference lists of all included studies and other reviews on the topic will be searched to identify any additional potentially relevant studies for inclusion. The Cochrane database and the Journal of Ageing Research Reviews will also be searched to identify reviews in similar areas.
Search strategy
Search terms are based on terms from existing reviews, for example, Bahar-Fuchs et al. [22], Kelly et al. [24], Simon et al. [25], Li et al. [57]. The MEDLINE (Ovid) search strategy is detailed in the Appendix section. This search strategy will be tailored to the thesaurus or controlled vocabulary and search syntax of each database. Publications will be limited to human studies, published in English peer-reviewed journals, between March 2009 and March 2019. Results from the database searches will be exported and managed in an Endnote reference management software. The searches will be re-run just before final analyses and further studies retrieved for inclusion if found.
Study selection and data extraction process
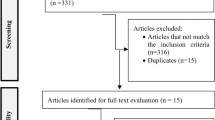
Two independent reviewers (NT and KL) will be involved in the study selection and data extraction. The study selection process will be in accordance with the PRISMA guidelines (Fig. 1). A data extraction form will be developed and piloted independently by two reviewers (NT and KL) on 10% of the identified studies prior to use and modified as required.
During initial screening, all papers with study titles and abstracts viewed as potentially eligible by at least one of the two reviewers will be retained for full review. A full review will be conducted by the two reviewers independently. Reasons for inclusion and exclusion will be recorded. Once eligible papers have been identified, data will be extracted independently by the two reviewers. Disagreements relating to eligibility and differences in data extraction will be resolved by discussion between the two reviewers to reach a consensus. A third reviewer (MB) will resolve any difference identified between the two reviewers.
When information is not available within the selected studies, contacting the authors via email will be attempted. If the information is not available after this process, the most conservative estimates will be made using available data, i.e., at the lower 95% confidence interval.
Methodological critique of evaluation research
The methodological quality of the included studies will be assessed using the Physiotherapy Evidence Database (PEDro) scale [46]. The risk of bias assessment will be critiqued independently by two reviewers (NT and KL); a third reviewer (MB) will resolve any differences in opinion.
Data extraction
The following data will be extracted:
-
1.
Participant information: sample size, mean age, diagnosis and diagnostic criteria utilised and baseline cognitive score if indicated.
-
2.
Methods of each study: study design, treatment setting and methodological limitations reported.
-
3.
Type of interventions: aim of intervention, type of cognitive intervention, duration of treatment (duration of sessions, frequency of sessions, period of intervention, total hours of intervention), method of intervention delivery and description of control group intervention.
-
4.
Outcome measures: performance of IADL at pre- and post-intervention period and post-intervention follow-up if data is available.
-
5.
Results of the studies: impact (if any) in the performance of IADL for experimental and control groups.
If identified studies include mixed cohorts (including healthy adults, MCI or dementia, or combining with people younger than 60), an attempt will be made to contact the corresponding author to request results for just the eligible participants. If not, these studies will be excluded from the review.
Synthesis and analysis of results
A narrative synthesis of the findings will be used to report outcomes of all included studies. This synthesis will be formatted around study type, sample size, participant characteristics, outcomes and outcome measures. The context of intervention on type, quantity, frequency and/or duration of therapy will be described. ‘Summary of findings’ tables will be created to provide key information regarding evidence quality, a summary of available data on outcomes and the degree of the effectiveness of interventions. The synthesis of findings will be in accordance with the Centre for Reviews Dissemination [58]. The Economic and Social Research Council guidance report [59, 60] will be used as a framework for a narrative synthesis. This framework consists of four key elements: (1) the development of a theory of how the intervention works, why and for whom, (2) the development of a preliminary synthesis of findings of included studies, (3) the identification of relationships within and between studies and (4) the assessment of the strength of the synthesis.
Measures of treatment effectiveness
Each study and outcome measure will be assessed for suitability for meta-analysis. Two reviewers (NT and KL) will evaluate and identify the major outcome measure that represents the main outcome of each study for meta-analysis. The treatment effects, based on pooled data from individual studies, will be recorded. Means and standard deviations (SDs) or medians at pre-, post-intervention and follow-up assessments will be extracted from each study. If the means and SDs or medians are not available, the corresponding author will be contacted for the available data. If further information is not available, medians will be used to replace means, and baseline SD will be used as an estimate of SD at follow-up. If the required data cannot be retrieved, the study will be excluded from the meta-analysis.
A separate analysis will be performed on studies using cognitive training, cognitive rehabilitation and cognitive stimulation. Depending on available data extracted from each study, identical and non-identical outcome measures will be combined for analysis with the standardised mean differences (SMD). In addition, the p value and 95% confidence intervals (CI) will be reported. If both categorical and numeric measures are used in the studies, two separate meta-analyses will be conducted. Heterogeneity will be assessed using the I2 statistic. Meta-analysis with an I2 > 40% will be considered to have substantial heterogeneity, and appropriate warnings will be given against overinterpretation of these results [61]. The sample size will be weighted downwards by the estimated design effect. The design effect will be estimated using the intra-class correlation coefficient (ICC) if provided. If the ICC is not provided, it will be estimated from the wider literature. The effect size of individual outcome measures will be calculated using Hedge’s g, with included adjustments for small sample size. The analysis will be performed using the ‘metafor’ package in R software, where the random effect model with 95% CI will be used [62].
The robustness and generalisability of the results will be explored by a variety of sensitivity analyses such as excluding the lower quality studies and studies from less developed or developing countries. An experienced statistician will assist with the completion of the meta-analysis.
Confidence in cumulative evidence
The quality of evidence for primary outcomes from each of the studies included in the review will be assessed using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach [63] by the first author (NT). A second author (KL) will verify the ratings; any disagreements will be discussed and reconciled with a third author (MB). The meta-analysis will be assessed in relation to the quality of the evidence scored in the five domains specified within GRADE: limitations in study design and/or execution (risk of bias), inconsistency of results, indirectness of evidence, imprecision of results and publication bias [63]. The quality of each study will be rated as high, moderate, low or very low according to the level of confidence in where the effect lies in relation to the estimated effect.
Ethics and dissemination
Ethical approval is not required for this study, as this systematic review did not directly or indirectly involve human participants. Data will be extracted from publicly available published literature, and the analysis is secondary to this. Findings from this systematic review will be submitted as a manuscript for peer review in an appropriate journal. Findings will also be presented to clinicians and researchers at relevant conferences.
Amendments
If amendments to the protocol outlined are required, the date of the amendment, the change required and rationale for the change will be documented in a protocol addendum and in the final report of the systematic review.
Discussion
Dementia is an overarching term used to describe a group of diseases associated with an observable decline in cognitive functioning, and individuals living with these conditions often have difficulty with daily activities that affect their independent living. Due to the multiple clinical presentations and underlying etiologies associated with dementia and MCI, there is currently no curative treatment available for the underlying diseases that cause these syndromes [64]. This systematic review and meta-analysis will determine the effectiveness of cognitive interventions in maintaining or improving the performance of IADL in individuals with MCI or mild dementia. It is anticipated that the dissemination of results will inform rehabilitation professionals on the most effective cognitive interventions for clinical practice. It will potentially provide substantial benefit to both people living with MCI or dementia and the health care system by keeping more people living in the community rather than full-time residential care and allowing those in full-time residential care to require less intensive support. Moreover, it can inform and encourage the development of policies and guidelines for the support of these individuals. Results and policy recommendations will be presented at rehabilitation conferences and policy forums. In addition, our description of all recent research in this topic area will identify if and where further research is required.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence intervals
- IADL:
-
Instrumental Activities of Daily Living
- MCI:
-
Mild cognitive impairment
- P value:
-
Probability value
- PEDro:
-
Physiotherapy Evidence Database
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- SD:
-
Standard deviations
- SMD:
-
Standardised mean differences
References
American Occupational Therapy Association [AOTA]. Occupational therapy practice framework: domain and process. Am J Occup Ther. 2014;68(Supplement_1):S1–S48.
Perneczky R, Pohl C, Sorg C, Hartmann J, Komossa K, Alexopoulos P, et al. Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing. 2006;35(3):240–5.
Perneczky R, Pohl C, Sorg C, Hartmann J, Tosic N, Grimmer T, et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int J Geriatr Psychiatry. 2006;21(2):158–62.
Njegovan V, Man-Son-Hing M, Mitchell SL, Molnar FJ. The hierarchy of functional loss associated with cognitive decline in older persons. J Gerontol Ser A Biol Med Sci. 2001;56(10):M638–M43.
Bennett HP, Piguet O, Grayson DA, Creasey H, Waite LM, Lye T, et al. Cognitive, extrapyramidal, and magnetic resonance imaging predictors of functional impairment in nondemented older community dwellers: the Sydney older person study. J Am Geriatr Soc. 2006;54(1):3–10.
de Paula JJ, Diniz BS, Ebicalho MA, Ealbuquerque M, Enicolato R, Moraes EN, et al. Specific cognitive domains and symptoms of depression as predictors of activities of daily living in older adults with heterogeneous cognitive backgrounds. Front Aging Neurosci. 2015;7.
Williams K, Kemper S. Exploring interventions to reduce cognitive decline in aging. J Psychiatr Nurs Ment Health Serv. 2010;48(5):42–51.
Hughes T, Chang C-C, Bilt J, Snitz B, Ganguli M. Mild cognitive deficits and everyday functioning among older adults in the community: the Monongahela-Youghiogheny Healthy Aging Team study. Am J Geriatr Psychiatry. 2012;20(10):836–44.
Niccolai LM, Triebel KL, Gerstenecker A, McPherson TO, Cutter GR, Martin RC, et al. Neurocognitive predictors of declining financial capacity in persons with mild cognitive impairment. Clin Gerontol. 2017;40(1):14–23.
Reppermund S, Sachdev PS, Crawford J, Kochan NA, Slavin MJ, Kang K, et al. The relationship of neuropsychological function to instrumental activities of daily living in mild cognitive impairment. Int J Geriatr Psychiatry. 2011;26(8):843–52.
Pérès K, Helmer C, Amieva H, Orgogozo JM, Rouch I, Dartigues JF, et al. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. J Am Geriatr Soc. 2008;56(1):37–44.
Jefferson AL, Cahn-Weiner D, Boyle P, Paul RH, Moser DJ, Gordon N, et al. Cognitive predictors of functional decline in vascular dementia. Int J Geriatr Psychiatry. 2006;21(8):752–4.
Dodge HH, Du Y, Saxton JA, Ganguli M. Cognitive domains and trajectories of functional independence in nondemented elderly persons. J Gerontol Ser A Biol Med. Sci. 2006;61:1330–7.
Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23(2):168.
Cahn-Weiner DA, Farias ST, Julian L, Harvey DJ, Kramer JH, Reed BR, et al. Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc. 2007;13(5):747–57.
Farias S, Cahn-Weiner DA, Harvey DJ, Reed BR, Mungas D, Kramer JH, et al. Longitudinal changes in memory and executive functioning are associated with longitudinal change in instrumental activities of daily living in older adults. Clin Neuropsychol. 2009;23(3):446–61.
Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, et al. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7(1):7–17.
Williams KN, Kemper S. Interventions to reduce cognitive decline in aging. J Psychosoc Nurs Ment Health Serv. 2010;48(5):42–51.
Liu KPY, Chan CCH, Chu MML, Ng TYL, Chu LW, Hui FSL, et al. Activities of daily living performance in dementia. Acta Neurol Scand. 2007;116(2):91.
Chaves G, Oliveira AM, Chaves JA, Forlenza OV, Aprahamian I, Nunes PV. Assessment of impairment in activities of daily living in mild cognitive impairment using an individualized scale. Arquivos de Neuro-Psiquiatria. 2016;74(7):549–54.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub; 2013.
Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2013;6:CD003260.
Faucounau V, Wu Y, Boulay M, Rotrou J, Rigaud A. Cognitive intervention programmes on patients affected by mild cognitive impairment: a promising intervention tool for MCI? The Journal of Nutrition. Health Aging. 2010;14(1):31–5.
Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev. 2014;15:28–43.
Simon SS, Yokomizo JE, Bottino CMC. Cognitive intervention in amnestic Mild Cognitive Impairment: a systematic review. Neurosci Biobehav Rev. 2012;36(4):1163–78.
Belleville S. Cognitive training for persons with mild cognitive impairment. Int Psychogeriatr. 2008;20(1):57–66.
Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300(15):1774–83.
Fisk J, Rockwood K. Outcomes of incident mild cognitive impairment in relation to case definition. J Neurol Neurosurg Psychiatry. 2005;76(8):1175–7.
Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61(9):1179–84.
Ritchie LJ, Tuokko H. Clinical decision trees for predicting conversion from cognitive impairment no dementia (CIND) to dementia in a longitudinal population-based study. Arch Clin Neuropsychol. 2010;26(1):16–25.
Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. The Lancet. 2006;367(9518):1262–70.
Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753–72.
Lopez OL. Mild cognitive impairment. Continuum: Lifelong Learning in Neurology. 2013;19(2 Dementia):411.
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–92.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8.
Yanhong O, Chandra M, Venkatesh D. Mild cognitive impairment in adult: a neuropsychological review. Ann Indian Acad Neurol. 2013;16(3):310.
Ellis KA, Szoeke C, Bush AI, Darby D, Graham PL, Lautenschlager NT, et al. Rates of diagnostic transition and cognitive change at 18-month follow-up among 1,112 participants in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL). Int Psychogeriatr. 2014;26(4):543–54.
World Health Organization. Dementia 2019 [Available from: https://www.who.int/news-room/fact-sheets/detail/dementia
World Health Organization. World Report on Ageing and Health 2015. Available from: http://apps.who.int/iris/bitstream/10665/186463/1/9789240694811_eng.pdf?ua=1.
Australian Institute of Health and Welfare. Older Australia at a glance 2017 [Available from: https://www.aihw.gov.au/reports/older-people/older-australia-at-a-glance/contents/demographics-of-older-australians/australia-s-changing-age-and-gender-profile.
Australian Institute of Health and Welfare. Dementia in Australia Canberra, ACT: AIHW; 2012.
Australian Bureau of Statistics. Population projections, Australia, 2012 (base) to 2101. Canberra: ACT: ABS; 2013.
Jean L, Bergeron M-È, Thivierge S, Simard M. Cognitive intervention programs for individuals with Mild Cognitive Impairment: systematic review of the literature. Am J Geriatr Psychiatry. 2010;18(4):281–96.
Productivity Commission. Economic implications of an ageing Australia: research report. ACT: Commonwealth of Australia: Canberra; 2005.
Brown L, Hansnata E, La HA. Economic cost of dementia in Australia 2017. Available from: https://www.dementia.org.au/files/NATIONAL/documents/The-economic-cost-of-dementia-in-Australia-2016-to-2056.pdf.
Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. 2009;339(7716):332.
Organization WH. International statistical classification of diseases and related health problems: World Health Organization; 2004.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer’s disease: report of the NINCDS-ADRDA work group⋆ under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939–44.
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72.
Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811.
Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal Intern Med. 2004;256(3):183–94.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer’s & Dementia. 2011;7(3):270–9.
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6.
Littbrand H, Stenvall M, Rosendahl E. Applicability and effects of physical exercise on physical and cognitive functions and activities of daily living among people with dementia: a systematic review. Am J Phys Med Rehabil. 2011;90(6):495–518.
Cove J, Jacobi N, Donovan H, Orrell M, Stott J, Spector A. Effectiveness of weekly cognitive stimulation therapy for people with dementia and the additional impact of enhancing cognitive stimulation therapy with a carer training program. Clin Interv Aging. 2014;9:2143.
Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet. 2015;385(9984):2255–63.
Li H, Li J, Li N, Li B, Wang P, Zhou T. Cognitive intervention for persons with mild cognitive impairment: a meta-analysis. Ageing Res Rev. 2011;10(2):285–96.
Tacconelli E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect Dis. 2010;10(4):226.
Rodgers M, Sowden A, Petticrew M, Arai L, Roberts H, Britten N, et al. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews. Evaluation. 2009;15(1):49–73.
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, Britten, N, Roen, K, Duffy, S. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme; 2006.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; 2011.
Wolfgang V. Conducting meta-analyses in r with the metafor package. J Stat Softw. 2010;36(3):1–48.
Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38.
Petersen RC. Mild cognitive impairment: current research and clinical implications. Seminars in neurology. USA: Thieme Medical Publishers, Inc; 2007.
Acknowledgements
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NT, KL, MB, RB, KC and PF were involved in the study design. NT, KL and KC developed the search strategies. NT and KL drafted the manuscript of the protocol. MB, RB, KC and PF revised the manuscript. NT and KL will screen the potential studies, extract the data and assess quality. Any discrepancies identified will be cross-checked by MB. PF and NT will perform the meta-analysis. KL is the guarantor of the review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Database: Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) without Revisions <1996 to February 09, 2018> Search Strategy:
-----------------------------------------------------------------
-
1.
exp Dementia/ or exp Cognitive Dysfunction/ or exp Alzheimer Disease/or exp Cognition Disorders/ (157088)
-
2.
((mild adj dementia) or MCI or dementia).tw. (81709)
-
3.
((cognitive adj2 dementia) or CIND).tw. (1944)
-
4.
memory disorders/ or exp amnesia/ (19643)
-
5.
((age-associated adj2 impairment) or AAMI).tw. (554)
-
6.
((age-related adj2 impairment) or (memory adj impairment) or (memory adj decline) or (memory adj loss) or (impaired adj memory)).tw. (13051)
-
7.
(alzheimers or (cognitive adj decline) or (memory adj decline)).tw. (102555)
-
8.
1 or 2 or 3 or 4 or 5 or 6 or 7 (238990)
-
9.
exp Cognitive Therapy/ or (cognitive adj therap$).tw. (22154)
-
10.
(cognitive and (intervention or training or techniques or restoration or retraining or re-training or stimulation or rehabilitation or remediation)).tw. (48052)
-
11.
exp Neurological Rehabilitation/ or Rehabilitation/ (15310)
-
12.
exp Mental Recall/ or (mental adj stimulation).tw. (20456)
-
13.
(task adj2 training).tw. (1275)
-
14.
exp Occupational Therapy/ or (occupational adj rehabilitation).tw. (6356)
-
15.
((sensory adj stimulation) or (reminiscence adj therapy)).tw. (1854)
-
16.
exp "Imagery (Psychotherapy)"/ or (mental adj imagery).tw. (2438)
-
17.
(skill and (acquisition or retention)).tw. (3582)
-
18.
exp Learning/ or (memory adj training).tw. (226476)
-
19.
(memory and (encoding or retrieval)).tw. (15907)
-
20.
((task adj2 training) or (functional adj task)).tw. (1586)
-
21.
((guided adj imagery) or (motor adj imagery)).tw. (2513)
-
22.
Visual Perception/ or Cues/ (57601)
-
23.
(visualisation or cues).tw. (55718)
-
24.
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 (372351)
-
25.
exp "Activities of Daily Living"/ or ADL.tw. (51146)
-
26.
((Instrumental adj3 living) or (activities adj living) or IADL).tw. (2617)
-
27.
(functional and (performance or ability or status)).tw. (136152)
-
28.
(daily and (task or activities)).tw. (47564)
-
29.
((complex adj activities) or (task adj performance) or (day adj2 activities)).tw. (9592)
-
30.
25 or 26 or 27 or 28 or 29 (216937)
-
31.
8 and 24 and 30 (4691)
-
32.
limit 31 to (english language and yr="2008 -Current") (3129)
-
32.
limit 32 to randomized controlled trial (370)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tulliani, N., Bissett, M., Bye, R. et al. The efficacy of cognitive interventions on the performance of instrumental activities of daily living in individuals with mild cognitive impairment or mild dementia: protocol for a systematic review and meta-analysis. Syst Rev 8, 222 (2019). https://doi.org/10.1186/s13643-019-1135-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-019-1135-0