Abstract
Background
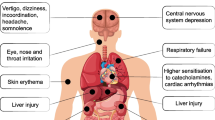
S-lon® (S) is a locally produced polyvinyl chloride-based solvent cement. It is a clear, slightly viscous liquid. Other constituents include 1-cyclohexanone, 3-butanone, and 1-acetone. It is used ubiquitously for building construction in Sri Lanka. Although the clinical effects of the compound have not yet been ascertained, the constituents have been implicated in neurotoxicity, respiratory tract, eye and skin irritation, and delayed liver and renal injury.
Case description
A 42-year-old South Asian male presented following self-ingestion of S. His vital parameters were stable and initially managed symptomatically. A few hours later, he developed central nervous system depression and stridor requiring elective intubation. Examination of the upper airway revealed inflammation and edema. He was sedated and ventilated, and intravenous dexamethasone was administered. Attempts at removal of the nasogastric tube after extubation on day 3 failed. The patient had to be reintubated and sedated owing to extreme agitation not responding to routine doses of sedatives. The nasogastric tube had been amalgamated after reacting with S, forming a solid clump, later found after removal. The posterior pharynx and nasopharynx were packed and later removed before extubation. The patient made a full recovery and was transferred to the ward on day 5.
Conclusion
Ingestion of a sufficient quantity of S could result in gut absorption with central nervous system depression, coma, and even death. No antidote is available for toxicity, and management is largely supportive. As witnessed in our patient, chemical laryngitis and upper airway inflammation may lead to upper airway obstruction. Chemical reactions with medical equipment may lead to unforeseen outcomes.
Similar content being viewed by others
Introduction
S-lon® (S) is a locally produced polyvinyl chloride (PVC)-based solvent cement [1]. It is highly flammable and comes as a clear, slightly viscous liquid. Other constituents include 1-cyclohexanone, 3-butanone, and 1-acetone. It is used ubiquitously for building construction in Sri Lanka. Although the clinical effects of this compound have not yet been ascertained, the constituents have been implicated in neurotoxicity, respiratory tract, eye and skin irritation, and delayed liver and renal injury [1]. Ingestion of a sufficient quantity could result in gut absorption, resulting in central nervous system (CNS) depression, coma, and even death [1]. Deliberate self-ingestion is extremely rare. We report herein a case where a 42-year-old South Asian male presented following self-ingestion of S with subsequent CNS depression requiring elective intubation and difficult removal of nasogastric tube (NGT) owing to amalgamation requiring ear, nose, and throat (ENT) input.
Case description
A 42-year-old South Asian male, a father of two, presented to the emergency treatment unit of a District General Hospital in Sri Lanka following deliberate self-ingestion of S following an attempted suicide. No other toxins were found in the vicinity. His past medical, surgical, and allergy history had been insignificant. Admission was within 90 min following the ingestion. On admission, his airway was patent with equal bilateral air entry without added sounds, respiratory rate was 16 breaths per minute with a shallow breathing pattern, and capillary O2 saturation was 92% in room air. The pulse rate was 110 bpm; capillary refill was less than 2 s with a blood pressure of 140/85 mmHg. GCS was 11/15 (E-3, V-3, M-5), and bilateral pupils were 3 mm in size and equally reacted to light. Fundoscopy did not reveal papilledema. The capillary blood glucose level was 130 mg/dl.
The patient was managed symptomatically with face mask O2 of 10 l/min. He was catheterized, and an input–output chart was generated. A 16G NGT was inserted, and he was treated with activated charcoal. Over 90 min, 3% saline 150 ml was given to alleviate possible cerebral edema. Blood was sent for investigations including venous blood gas. The latter yielded normal acid–base status, electrolytes, and lactate. An electrocardiogram revealed sinus tachycardia with a rate of about 120 bpm. X-ray of the chest was normal. The patient’s GCS gradually deteriorated, and simultaneously airway patency was reduced with stridor. He was intubated under direct laryngoscopy following rapid sequence induction with IV propofol 2 mg/kg and IV suxamethonium 2 m/kg and cricoid pressure by an anesthetist. The pharynx appeared inflamed. Intravenous dexamethasone 8 mg stat dose was administered. Arterial blood gas after the intubation revealed a pH of 7.43, PCO2 27 mmHg, PO2 241 mmHg with lactate of 4.3 mmol/l and base excess of −5 mmol/l. A 500 ml 0.9% saline bolus was administered, and the maintenance fluid was increased to 120 ml/h.
The patient was admitted to the intensive care unit (ICU) for further management and monitoring. His initial blood investigations are illustrated in Table 1.
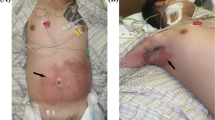
At the ICU, the patient was sedated and paralyzed to carry out lung and neuroprotective ventilation. Supportive care was given with stress ulcer and thromboprophylaxis. ENT referral was performed to assess the laryngeal and pharyngeal areas under fiber-optic laryngoscopy (FOL), and the findings were compatible with chemical laryngitis with mildly inflamed glottis, epiglottis, mildly edematous subglottic area, and vestibule of the larynx. Regular intravenous dexamethasone was administered. The patient was kept nil by mouth on the first 2 days, and NG feeding commenced after 48 h. His liver enzymes gradually dropped to normal levels. On day 3, sedation was stopped. The GCS had improved to 10/10. Successful extubation of the trachea was performed. Repeat FOL revealed that the laryngopharyngeal edema had now resolved. The patient appeared anxious and did not tolerate the NGT. As the patient was tolerating clear fluids via the oral route, the decision was made for its removal. Removal failed as the NGT was found to be lodged in the nasopharynx and attempts at pushing it back toward the oropharynx were unsuccessful. The patient became extremely anxious not responding to routine doses of sedatives. For the safety of the patient and the staff, he was reintubated and sedated. Subsequently, the NGT was removed by the ENT surgeon; the procedure was mildly traumatic with mild bleeding from the nasopharynx. The nasopharynx was packed with an adrenaline-soaked gauze. A Foley catheter was inserted, and the bulb was kept inflated for tamponade effect. The distal end of the NGT had formed a clump after reacting with S, which had led to the initial failure in removal (Fig. 1). The ENT team’s advice was to keep the pack for 48 h, after which the ENT team could remove the pack and the catheter. FOL was repeated, and inflammation of the laryngopharyngeal area had improved with the absence of significant bleeding. The leak test was repeated and found to be positive.
The nasal pack was removed after 48 h, and the patient’s trachea was extubated. Oral feeding was gradually commenced. He was discharged to the ward on day 5. A psychiatry referral was done at the ward, and upper gastrointestinal (GI) endoscopy was arranged at a tertiary care center in 2 weeks. Toxicology studies were not carried out as these were not available freely in this low-resource setting.
Discussion
S is classified as a hazardous substance and Schedule 5 poison according to the manufacturer [1]. It consists of polyvinyl chloride polymer, and the main constituent is 1-cyclohexanone (25–70%) with a mixture of 3-butanone (1–15%) and 1-acetone (0–10%). Doses and plasma concentrations leading to toxicity have been studied in animals, while relevant human data for the product as a whole are not available [1]. It is found to cause toxic effects after exposure in humans that include dermal, eye, pulmonary, gastrointestinal, and nervous systems [2]. Cyclohexanone toxicity is rare in humans and animals used for experimental purposes [3]. Inhalation is the predominant route of toxicity and can lead to respiratory tract irritation and skin, eye irritation, and CNS depression in large quantities [4, 5]. Toxicity after ingestion of cyclohexanone-containing organic solvents has not been reported elsewhere, according to our knowledge, thus data on definitive management are lacking.
It is possible that our patient (initially managed in the emergency treatment unit) developed upper airway obstruction (indicated by the onset of stridor) owing to upper airway inflammation caused by S and subsequently developed hypoxia and hypercarbia resulting in reduced consciousness. The arterial blood gas analysis was performed after intubation, mechanical ventilation, and stabilization of the patient, which might not reflect the preceding parameters. Similarly, CNS depression directly due to the toxicity of S can also be a causative factor, although the risk is stated to be low following ingestion route [1].
According to the manufacturers, following acute ingestion, active vomiting is discouraged and measures should be taken to prevent aspiration in a patient who is vomiting [1]. Supportive therapy in the form of supplementary oxygen and correction of electrolytes is similarly advocated. Activated charcoal adsorbs nonpolar, poorly water-soluble compounds [6]. S is described as a partially miscible substance in water, thus the use of activated charcoal is justified in case of significant ingestion. As witnessed in our patient, any clinical features of respiratory tract inflammation and airway involvement indeed warrant heightened alertness for early identification and management of life-threatening compromise. In animal studies, cyclohexanone has been implicated in delayed liver, renal, and hematological derangements. It is thus prudent to monitor human exposures for an extended period.
S is commercially used as a bonding material for PVC tubing [1]. Interestingly, the NGT used in this patient was made of PVC. The ingested S likely led to the amalgamation of the distal end of the NGT. Owing to multiple distal side openings in the NGT, it would have been functional until removal. We came across a recent case report of a young male who presented with small bowel obstruction 8 months after ingestion of a chlorinated PVC solvent cement and was found to have an ileal perforation during open laparotomy [7].
Conclusion
S poisoning following ingestion is seldom reported in literature. Clinicians need to be aware of the related potential airway and CNS emergencies. We present herein probably the first ever case of poisoning following ingestion of S. Initial resuscitation, continued in-hospital monitoring, and multidisciplinary care resulted in successful management of uncommon and unanticipated clinical encounters and importantly a favorable outcome.
Availability of data and materials
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- S :
-
S-lon®
- PVC:
-
Polyvinyl chloride
- CNS:
-
Central nervous system
- NGT:
-
Nasogastric tube
- ENT:
-
Ear, nose, throat
- GCS:
-
Glasgow Coma Score
- FOL:
-
Fiber-optic laryngoscopy
References
Material Safety Data Sheet—S-lon Solvent Cement. S-lon Lanka (Pvt) Ltd. 2019. http://www.slon.lk/storage/app/uploads/public/5c6/553/807/5c655380761f5778136896.pdf. Accessed 3 Dec 2023.
Cyclohexane Incident Management. Public Health England. 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/566904/cyclohexane_incident_management.pdf. Accessed 3 Dec 2023.
Lee YH, Chung YH, Kim HY, Shin SH, Lee SB. Subacute inhalation toxicity of cyclohexanone in B6C3F1 mice. Toxicol Res. 2018;34(1):49–53. https://doi.org/10.5487/TR.2018.34.1.049.
New Jersey department of health. Hazardous substance fact sheet. 2009. http://www.nj.gov/health/eoh/rtkweb/documents/fs/0164.pdf/. Accessed 3 Dec 2023.
National Center for Biotechnology Information. PubChem compound summary for CID 7967, cyclohexanone. 2024. https://pubchem.ncbi.nlm.nih.gov/compound/Cyclohexanone. Accessed 13 Jan 2024.
Chacko B, Peter JV. Antidotes in poisoning. Indian J Crit Care Med. 2019;23(Suppl 4):S241–9. https://doi.org/10.5005/jp-journals-10071-23310.
Huzaifa M, Sistla SC, Kumari S. An unusual case of small bowel obstruction caused by chlorinated polyvinyl chloride solvent cement consumption. Indian J Surg. 2023;85(3):652–4.
Acknowledgements
The authors would like to recognize and acknowledge all the clinical and non-clinical staff who were involved in the management of the patient.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SM, TD, and CW managed the patient. All authors contributed to the manuscript in terms of planning, conception and design, writing and editing, and reading and approving the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marambahewa, S.R., Chandrasiri, D.A.C.T., Wijesekara, W.A.I.C. et al. Polyvinyl chloride solvent cement poisoning: a case report. J Med Case Reports 18, 155 (2024). https://doi.org/10.1186/s13256-024-04470-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04470-x