Abstract
Background
Patients infected with severe acute respiratory syndrome coronavirus (SARS-CoV-2) can develop severe illness necessitating intensive care admission. Critically ill patients are susceptible for the development of secondary bacterial infections. Due to a combination of virus- and drug-induced immunosuppression, critically ill patients with corona virus disease 2019 (COVID-19) may even have a higher risk of developing a secondary infection. These secondary infections can aggravate the severity of illness and increase the risk of death. Further research on secondary infections in COVID-19 patients is essential. Therefore, the objective of this study was to investigate the incidence and associated risk factors of secondary bacterial infections and to identify the most common groups of pathogens in critically ill COVID-19 patients.
Methods
This mono-center, retrospective observational cohort study was performed at the intensive care unit (ICU) of the Jessa Hospital, Hasselt, Belgium. All adult COVID-19 patients admitted to the ICU from 13th March 2020 until 17th October 2020, were eligible for inclusion in the study. Data from the resulting 116 patients were prospectively entered into a customized database. The resulting database was retrospectively reviewed to investigate three types of secondary bacterial infections (secondary pneumonia, bloodstream infections of unknown origin, catheter-related sepsis).
Results
Of 94 included patients, 68% acquired at least one of the studied secondary bacterial infections during their ICU stay. Almost two thirds of patients (65.96%, n = 62) acquired a secondary pneumonia, whereas 29.79% (n = 28) acquired a bacteremia of unknown origin and a smaller proportion of patients (14.89%, n = 14) acquired a catheter-related sepsis. Male gender (P = 0.05), diabetes mellitus (P = 0.03) and the cumulative dose of corticosteroids (P = 0.004) were associated with increased risk of secondary bacterial infection. The most common pathogens detected in the cultures of patients with secondary pneumonia were Gram-negative bacilli. Bacteremia of unknown origin and catheter-related sepsis were mostly caused by Gram-positive cocci.
Conclusion
This study confirms that the incidence of secondary bacterial infections is very high in critically ill COVID-19 patients. These patients are at highest risk of developing secondary pneumonia. Male gender, a history of diabetes mellitus and the administration of corticosteroids were associated with increased risk of secondary bacterial infection.
Similar content being viewed by others
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the ongoing pandemic of coronavirus disease (COVID-19). The spectrum of disease severity of patients infected with SARS-CoV-2 is very wide: from an asymptomatic carrier state to severe lower respiratory tract infection and critical illness with intensive care unit (ICU) requirement [1].
In general, critically ill patients are susceptible for development of secondary bacterial infections such as secondary pneumonia, bloodstream infection of unknown origin, and-catheter related sepsis. It has been suggested that critically ill patients infected with SARS-CoV-2 are even at higher risk of developing secondary infections due to a combination of virus- and drug-induced immunosuppression [2]. Secondary infections may lead to a lower discharge rate and higher mortality rate [3]. As a result, further research on secondary infections in COVID-19 patients is essential.
In literature, there is a wide variation in reported incidences and outcomes of secondary bacterial infections in critical ill COVID-19 patients. A recent systematic review reported an incidence of secondary infections ranging from 7% up to 51% in critically ill patients infected with SARS-CoV-2 [4]. The most common bacterial complication of COVID-19 was secondary pneumonia including ventilator-assisted pneumonia (VAP). A recent multicenter study described a cumulative incidence of VAP of 50% in patients with COVID-19 admitted to the ICU [5]. Data on bloodstream infections in critically ill COVID-19 patients are rather scarce. It has been suggested that bloodstream infections are the second most common secondary infection in critically ill COVID-19 patients with incidences ranging from 3.4 to 50% [6, 7].
Identification of the pathogens most often responsible for the development of secondary infections in critical ill COVID-19 patients generates new possibilities such as individually tailored empiric antibiotic therapy in those patients with early signs of a secondary infection. Furthermore, identification of possible risk factors associated with secondary bacterial infections may lead to development of new prevention strategies for secondary infections.
Therefore, the objective of this study was to investigate the incidence and possible risk factors of secondary bacterial infections and to identify the most common groups of pathogens in critically ill patients infected with SARS-CoV-2.
Methods
Patients and study design
This mono-center, investigator-initiated, longitudinal, retrospective observational cohort study was performed at the ICU of the Jessa Hospital, Hasselt, Belgium, after approval by the ethical committee of Jessa Hospital, Hasselt, Belgium on 14th April 2021 (2021-037) and registration on clinicaltrials.gov (NCT04877808). Written informed consent was waived considering the urgent need to collect data on the ongoing pandemic and the retrospective nature of this study. This study is reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement [8].
All adult patients, with acute hypoxemic respiratory failure due to diagnosed COVID-19 pneumonia admitted to the ICU of Jessa from 13th March 2020 until 17th October 2020, were eligible for inclusion in the study. In accordance with the World Health Organisation (WHO) protocol [9], laboratory confirmation of COVID-19 infection was defined as a positive result on polymerase chain reaction (PCR) assays of nasopharyngeal swab samples or on bronchoalveolar lavage. This resulted in the screening of 116 COVID-19 patients admitted to the ICU. All patients were treated according to the COVID-protocol of the Jessa hospital (Additional file 1: Appendix), based on the latest insights on COVID-19 at that time point [10]. Data from all patients were prospectively entered into a customized database that included medical history, demographic data, clinical parameters, and laboratory results. Scores of severity of illness, such as APACHE IV scores and Sequential Organ Failure Assessment (SOFA) score, were calculated on ICU admission. The following outcome measures were investigated: ICU mortality, need of mechanical ventilation and length of stay (LOS) in the ICU and hospital LOS. Following completion of the database, the data were retrospectively reviewed.
Diagnosis of secondary bacterial infection
The diagnosis of a secondary bacterial infection was based on clinical symptoms in combination with laboratory analyses. Secondary pneumonia was diagnosed when the patient developed clinical symptoms, positive radiologic signs and had positive laboratory-confirmed culture from the lower respiratory tract [4, 5]. The diagnosis of blood stream infection of unknown origin was based on clinical symptoms in combination with a positive blood culture, in the absence of a confirmed or suspected origin of the infection [6, 7]. Catheter-related sepsis was diagnosed in the case of clinical symptoms related to sepsis in combination with a positive culture originating from a catheter [7]. All positive cultures were further analyzed to identify the responsible pathogen.
Statistical analyses
Continuous data are shown as mean ± standard deviation (SD) and categorical data are presented as frequencies and percentages. Comparisons between groups were performed with the Student’s t-tests for normally distributed data and with Mann Whitney U test for not normally distributed data. Categorical variables were analyzed with a Chi-Square test or, if appropriate, with a Fisher’s exact test. Univariable logistic regression was used to investigate the association of possible risk factors (age, gender, BMI, diabetes mellitus, hypertension, smoking, APACHE IV score, SOFA score on admission, need for hemodynamic assist device, cumulative dose of corticosteroids administered in the ICU, and other immune suppressive therapies) and the acquisition of a secondary bacterial infection. A P-value < 0.05 was considered statistically significant.
Results
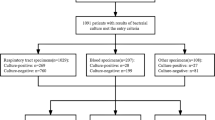
A STROBE flow chart depicting exclusion and inclusion of patients is shown in Fig. 1
In total, 22 patients were excluded. This resulted in data of 94 patients for final analysis.
Baseline characteristics
Baseline characteristics stratified for the occurrence of secondary infections are presented in Table 1.
Outcome measures
Outcome measures of the study population are presented in Table 2. There is a significant longer ICU stay (P < 0.001), longer hospital stay (P < 0.001), and longer need of mechanical ventilation (P < 0.001) in the cohort of patients diagnosed with a secondary bacterial infection.
Incidence of secondary bacterial infection
The incidence of all three different types of diagnosed secondary bacterial infections is visualized in Fig. 2. More than two third of included patients (n = 64, 68.1%) suffered from at least one of three predefined secondary bacterial infections (secondary pneumonia, bloodstream infection of unknown origin, catheter-related sepsis). Secondary pneumonia was the most frequently diagnosed secondary infection (n = 61, 64.9%). In 35 (57.4%) patients diagnosed with secondary pneumonia, the lung was the only secondary infection site. Blood stream infection of unknown origin was diagnosed in 28 (29.8%) patients. A smaller proportion of patients (14.9%, n = 14) acquired a catheter-related sepsis. Only 30 patients (31.9%) did not acquire one of the previous described secondary infections and 27 (28.7%) patients acquired more than one secondary infection.
Risk factors associated with the acquisition of a secondary infection
Univariate regression analyses showed that only male gender (P = 0.05), diabetes mellitus (P = 0.03), and the treatment with corticosteroids were associated with a higher likelihood of acquiring a secondary bacterial infection (P = 0.004) (Table 3).
Identification of most frequent pathogens
The identification of different types of pathogenic germs detected in the samples of the different infection sites are listed in Table 4. The most frequent types of pathogens identified in the cultures of patients with secondary pneumonia were Gram-negative bacilli (82%) followed by Gram-positive cocci (66%), Gram-negative cocci (24%), and Gram-positive bacilli (19%). Furthermore, in 12 patients (19.7%) with a secondary pneumonia, aspergillosis was detected in the cultures from the lower respiratory tractus. The most prevalent pathogens in bloodstream infection of unknown origin were Gram-positive cocci (89%) followed by Gram-negative bacilli (32%), Gram-negative cocci (18%), and Gram-positive bacilli (4%). In catheter related sepsis, Gram-positive cocci (79%) were the most prevalent whereas Gram-negative bacilli (36%), Gram-negative cocci (14%) and Gram-positive bacilli (7%) were less common.
Discussion
In this observational cohort study, we observed a very high incidence of 68% of secondary infections in critically ill patients infected with SARS-CoV-2. Secondary pneumonia (65%) was the most frequently diagnosed secondary infection, followed by bloodstream infection of unknown origin (30%) and catheter-related sepsis (15%). This study suggests that patients suffering from secondary infections may be at higher risk of longer ICU and hospital stay but not of ICU death.
Our results on the high incidence of secondary pneumonia are congruent with other cohort studies investigating secondary pneumonia in critically ill patients infected with SARS-CoV-2 [11]. Remarkably, the observed incidence is threefold higher than the incidence of secondary pneumonia in critically ill patients not infected with SARS-CoV-2 (13.5–23%) [12]. Other respiratory viral infections, such as seasonal/pandemic influenza, Middle East respiratory syndrome coronavirus and SARS-CoV-1 are also characterized by a high incidence of secondary pneumonia [13,14,15]. Bacterial infections responsible for secondary pneumonia originate from co-pathogens that can be found in the respiratory tract. In literature, it has been suggested that this type of pneumonia in critically ill patients may aggravate severity of illness and increases the risk of death [13,14,15,16,17]. In this study however, we couldn’t detect an increased risk of death in patients suffering from a secondary bacterial infection. It can be deduced from Table 2 that patients with a DNI code had a large impact on ICU mortality and other outcome variables. This impact most likely explains why we couldn’t detect an association between ICU mortality and acquisition of a secondary infection.
In this study cohort the most common type of pathogens responsible for secondary pneumonia were the Gram-negative bacilli, followed by Gram-positive cocci. This observation is consistent with what we would expect based on literature. A large multicenter study, performed in 36 European ICUs, identified Gram-negative bacilli, mainly Pseudomonas auruginosa, Enterobacter species, and Escherichia coli as the most common bacteria involved in secondary pneumonia in COVID-19 patients [5].
Besides secondary bacterial pneumonia, patients with a viral pneumonia in general, are prone for the development of invasive pulmonary aspergillosis, which has a negative impact on duration of hospitalization and mortality [18]. There is some data available, that suggest that critically ill COVID-19 patients have an even higher risk to develop invasive aspergillosis [19]. In this study cohort, the incidence of invasive aspergillosis in patients with a secondary pneumonia was 19.7%. This is in line with other European studies, which report a rate of 20–35% for invasive aspergillosis in critically ill COVID-19 patients with a secondary pneumonia [20, 21].
The incidence of blood stream infection and catheter-related sepsis was comparable with the incidence of secondary infection in critically ill patients not infected with SARS-CoV-2 [22]. The most common pathogens identified in blood cultures or catheter cultures from patients with a bloodstream infection or catheter-related sepsis were Gram-positive cocci. This is in line with the fact that Staphylococcus aureus is frequent a commensal on the skin and often responsible for blood stream infections and catheter-related infections.
The only identified risk factors associated with the acquisition of a secondary infection were a history of diabetes mellitus, the cumulative dose of corticosteroids administered in the ICU, and male gender. However, the investigated cohort was relatively small, thus it is presumable that other risk factors associated with the acquisition of a secondary infection could not be detected. The first identified risk factor, diabetes mellitus, is known to increase the incidence and severity of infectious diseases. Previous research indicates that diabetic patients with COVID-19 admitted to an ICU also have a higher reported mortality than non-diabetic patients [23]. The second factor that was identified as a possible risk factor was the total cumulative dose of corticosteroids administered in the ICU. This is logical, as corticosteroids are a known suppressor of the immune system. However, because of lack of information on timing, no firm conclusions can be drawn on the temporal association between corticosteroid therapy and secondary infection. Routine administration of corticosteroids was not part of the Jessa protocol at the early stage of this study period and were only administrated according to clinical decision of the attending intensivist.
Common policy was to only start corticoids in patients with signs of refractory systemic inflammatory disease without diagnosed secondary infection. Thus, it cannot be ruled out that a third bystander, i.e. more severe COVID-19 disease or pre-existing comorbidities, may have independently increased both cumulative dose of corticoid therapy and risk of secondary infection. Nevertheless, current guidelines advise the administration of corticosteroids to COVID-19 patients treated in the ICU [24]. Apparently, any possible risk associated with the administration of corticosteroids is outweighed by its positive effect on the course of the infectious disease itself. Male gender was also associated with increased risk of secondary bacterial infection. Obviously, this association might also be elicited by a third bystander.
This study has several limitations. First, the retrospective single-center design and the small number of patients included in this cohort negatively impacts the generalizability of our findings. Second, due to the small sample, only univariable analyses could be performed. To confirm the described possible associations further research is necessary. Third, information on identification of bacteria on a species level was not always provided by the department of microbiology. Therefore, we chose to present only broad categories of bacteria (Gram-positive/negative bacilli/cocci).
Conclusion
This study confirms that the incidence of secondary bacterial infections in critically ill patients infected with SARS-CoV-2 is very high. More specifically, these patients are at highest risk of developing secondary pneumonia, followed by bloodstream infection of unknown origin and catheter-related sepsis. Male gender, a history of diabetes mellitus and higher dosing of corticosteroids were associated with increased risk of secondary bacterial infection.
Availability of data and materials
Due to the applicable privacy regulation (GDPR) and Good Clinical Practices (GCP) legislation, the full underlying dataset supporting the study cannot be provided. This dataset contains potentially identifying information, for example age, BMI and comorbidities such as diabetes mellitus leading to a unique subject in the dataset. Therefore, descriptive statistics have been used for a general overview of our study population, and all other relevant information is provided in Table 1. Anonymized data is available on motivated request and can be send to: Prof. Dr. Björn Stessel; Stadsomvaart 11; 3500 Hasselt, Belgium; bjorn.stessel@jessazh.be; AND Jessa Ziekenhuis, Data Protection Officer (DPO); Stadsomvaart 11; 3500 Hasselt, Belgium; DPO@jessazh.be.
References
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Suarez-de-la-Rica A, Serrano P, De-la-Oliva R, Sánchez-Díaz P, Molinero P, Falces-Romero I, et al. Secondary infections in mechanically ventilated patients with COVID-19: an overlooked matter? Rev Esp Quimioter. 2021;34(4):330–6.
Zhang H, Zhang Y, Wu J, Li Y, Zhou X, Li X, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1958–64.
Grasselli G, Cattaneo E, Florio G. Secondary infections in critically ill patients with COVID-19. Crit Care. 2021;25(1):317.
Rouzé A, Martin-Loeches I, Povoa P, Makris D, Artigas A, Bouchereau M, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47(2):188–98.
Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):e13319.
Buetti N, Ruckly S, de Montmollin E, Reignier J, Terzi N, Cohen Y, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180–7.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9.
Laboratory testing for coronavirus disease (COVID-19) in suspected human cases. Interim guidance, World Health Organization. 19 March 2020. https://apps.who.int/iris/bitstream/handle/10665/331501/WHO-COVID-19-laboratory-2020.5-eng.pdf?sequence=1&isAllowed=y.
Alhazzani W, Møller MH, Arabi YM, Loeb M, Ng Gong M, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854–87.
Soriano MC, Vaquero C, Ortiz-Fernández A, Caballero A, Blandino-Ortiz A, de Pablo R. Low incidence of co-infection, but high incidence of ICU-acquired infections in critically ill patients with COVID-19. J Infect. 2021;82(2):e20–1.
Melsen WG, Rovers MM, Groenwold RH, Bergmans DC, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–71.
Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–61.
Joseph C, Togawa Y, Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respir Viruses. 2013;7 Suppl 2(Suppl 2):105–13.
Zahariadis G, Gooley TA, Ryall P, Hutchinson C, Latchford MI, Fearon MA, et al. Risk of ruling out severe acute respiratory syndrome by ruling in another diagnosis: variable incidence of atypical bacteria coinfection based on diagnostic assays. Can Respir J. 2006;13(1):17–22.
Esper FP, Spahlinger T, Zhou L. Rate and influence of respiratory virus co-infection on pandemic (H1N1) influenza disease. J Infect. 2011;63(4):260–6.
Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh YH, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10(5):394–403.
Schauwvlieghe A, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–92.
Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R, et al. COVID-19 associated pulmonary aspergillosis (CAPA)-from immunology to treatment. J Fungi (Basel). 2020;6(2):91.
Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8(6):e48–9.
van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132–5.
Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864–8.
Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354.
Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219–34.
Acknowledgements
This study is part of the Limburg Clinical Research Program (LCRP) UHasselt-ZOL-Jessa, supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital.
Funding
This study was funded solely by department funding.
Author information
Authors and Affiliations
Contributions
BS and JD conceived of the study. BS, AD and JD initiated the study design and SV, IC, LG and ID helped with implementation. LB was responsible for the statistical analyses. AD, LG, SV and IC were responsible for the data collection. BS, ID, AD and JD were responsible for the data interpretation. All authors were responsible for the writing of the paper and approved the final manuscript. AD and SV contributed equally to this manuscript and are therefore shared first author. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is approved by the ethical committee of Jessa Hospital, Hasselt, Belgium on 14th April 2021 (2021-037) and registered on clinicaltrials.gov (NCT04877808). Written informed consent was waived in light of the urgent need to collect data in the ongoing pandemic. This study was carried out in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
We declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Standard treatment regimen of COVID-19 patients admitted to the ICU.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
De Bruyn, A., Verellen, S., Bruckers, L. et al. Secondary infection in COVID-19 critically ill patients: a retrospective single-center evaluation. BMC Infect Dis 22, 207 (2022). https://doi.org/10.1186/s12879-022-07192-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07192-x