Abstract
Background
Bloodstream infections (BSI) caused by carbapenem-resistant K. pneumoniae (CRKP) are associated with high rates of morbidity and mortality. Early identification of patients at highest risk is very important. The aim of this study was to describe the clinical characteristics and mortality of K. pneumoniae BSI and to identify risk factors associated with CRKP BSI among paediatric patients.
Methods
From January 2011 to December 2014, a retrospective case-control study was conducted at Beijing Children’s Hospital, China. Risk factors for CRKP BSI and for K. pneumoniae BSI-related death were evaluated. Patients with BSI caused by K. pneumoniae were identified from the microbiology laboratory database. Data regarding demographic, microbiological and clinical characteristics, therapy and outcome were collected from the medical records.
Results
A total of 138 patients with K. pneumoniae BSI were enrolled, including 54 patients with CRKP BSI and 84 patients with carbapenem-susceptible K. pneumoniae (CSKP) BSI. Most of the BSI (114; 82.6%) were healthcare-associated, while the rest (24; 17.4%) were community-acquired. Hematologic malignancies (odds ratio (OR):4.712, [95% CI: 2.181–10.180], P < 0.001) and previous cephalosporin administration (OR: 3.427, [95% CI: 1.513–7.766], P = 0.003) were found to be associated with the development of CRKP BSI. 28-day mortality of K. pneumoniae BSI was 8.7%. Mechanical ventilation (OR:9.502, [95% CI: 2.098–43.033], P = 0.003), septic shock (OR:6.418, [95% CI: 1.342–30.686], P = 0.020), and isolation of CRKP (OR:9.171, [95% CI: 1.546–54.416], P = 0.015) were independent risk factors for 28-day mortality of K. pneumoniae BSI.
Conclusion
Hematologic malignancies and previous cephalosporin administration were associated with the development of CRKP BSI, while mechanical ventilation, septic shock and CRKP infection were independent mortality predictors for K. pneumoniae BSI. More attention should be paid to CRKP BSI in the paediatric population.
Similar content being viewed by others
Background
During the last decade, carbapenem resistant K. pneumoniae (CRKP) has spread worldwide, including to China [1, 2]. This organism is rapidly becoming a major threat to public health because of the limited therapeutic options and the high morbidity and mortality. Previous studies found that mortality rates ranged from 30 to 50% for infections caused by CRKP [3,4,5,6]. In China, this pathogen became endemic in many regions; the data from CHINET (an antimicrobial resistance surveillance network in China) surveillance regarding bacterial resistance demonstrated a marked increasing trend of CRKP between 2005 and 2014, with the rate rising from 2.4 to 13.4% [7].
CRKP can cause many types of infections, including pneumonia, urinary tract infections, bloodstream and intra-abdominal infections [8, 9]. Bloodstream infection (BSI) is the most important infection, with a high risk of mortality [10]. At its worst, with broad-spectrum antibiotic resistance, treatment options for BSI caused by CRKP are very limited [11, 12].
Because there are fewer therapeutic options in children [13, 14], the treatment of CRKP BSI is a real challenge for the paediatrician. The keys to success in preventing and treating CRKP infections are the implementation of infection control measures and early detection of patients at highest risk [14]. In this context, recognizing the risk factors associated with the development of CRKP BSI is of great importance when considering treatment options. Many studies have explored risk factors associated with carbapenem resistance among adults, including use of medical devices, previous antibiotic exposure, and ICU admission [14,15,16]. However, data regarding risk factors among children remain limited. Thus, the purpose of this study was to evaluate risk factors associated with CRKP BSI and mortality of K. pneumoniae BSI among paediatric patients as well as to describe clinical characteristics of K. pneumoniae BSI.
Methods
Study design and patients
This study was conducted at Beijing Children’s Hospital (a 970-bed tertiary paediatric hospital in China with an average of 70,000 admissions per year) between January 2011 and December 2014. Patients (from birth to 18 years of age) with confirmed K. pneumoniae BSI were included. A K. pneumoniae BSI was defined as the presence of at least once positive blood culture with concomitant signs and symptoms of infection according to established criteria [17, 18]. K. pneumoniae BSI cases were identified from the microbiology laboratory database. Only the first episode of K. pneumoniae BSI was included. Patients with polymicrobial BSIs or whose medical records were incomplete were excluded. CRKP was defined as minimal inhibition concentration (MIC) for imipenem or meropenem ≥ 4 μg/mL, or isolates were confirmed to produce carbapenemase (https://www.cdc.gov/hai/pdfs/cre/cre-guidance-508.pdf). To assess risk factors for CRKP BSI, a retrospective case-control study was performed. The CRKP group (case group) consisted of patients with CRKP BSI. Patients with BSI due to carbapenem-susceptible K. pneumoniae (CSKP) were defined as the CSKP group (control group).
The study was approved by the Ethics Committee of Beijing Children’s Hospital, Capital Medical University. Informed consent was waived because this study did not cause injury to patients.
Data collection and definitions
The data collected included information regarding demographics, underlying diseases, hospitalization, intensive care unit (ICU) admission, antibiotic therapy, intravascular catheter use, bacterial infections, and immunosuppressive therapy in the 90 days prior to the date of BSI onset; length of hospitalization, microbiological data, antimicrobial therapies, and patient outcomes were also collected.
BSI onset was defined as the collection date of a positive blood culture. BSI was classified as healthcare-related (HCR) or community-acquired (CA). Positive blood cultures obtained from patients who were hospitalized ≥ 48 h, or positive blood cultures obtained from patients who were hospitalized < 48 h but had been hospitalized in the previous six months, were defined as HCR-BSI; CA-BSI were defined as positive blood cultures obtained from patients who were hospitalized < 48 h without having been hospitalized in the previous six months [17]. Neutropenia was defined as absolute neutrophil count (ANC) lower than 500 cells/mm3 [18]. Therapy with one or more antimicrobial drugs within ≤ 24 h from BSI onset was defined as empirical antimicrobial therapy; treatment with antimicrobials after the susceptibility results became available was defined as definitive therapy [17]. The active antibiotic agent was defined as MIC within the susceptible range [19]. Appropriate antimicrobial therapy refers to the administration of the in vitro active agent, while inappropriate therapy was defined as treatment without active drugs [20]. The final outcome was determined as survival and all-cause death at 28 days after the date of BSI onset.
Microbiological methods
The Vitek 2 system (bioMérieux, Marcy l’Etoile, France) and the Phoenix100 automated system (Becton Dickinson, Spark, MD, USA) were used for isolate identification. The MIC values for tested antimicrobial agents were determined by an automated broth microdilution method (Becton Dickinson, Spark, MD, USA). The results were interpreted according to CLSI criteria (CLSI2014) [20]. For colistin, the results were interpreted in accordance with European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints (version 6.0). Polymerase chain reaction (PCR) testing was performed for detection of carbapenemase genes using a previously described method [21].
Statistical analysis
Categorical variables were presented as numbers and percentages. Continuous variables were presented as the mean and standard deviation (SD) (normally distributed) or median and interquartile range(IQR) (non-normally distributed). Categorical variables were compared using the chi-square or Fisher’s exact tests. Continuous variables were compared by Student’s t test or Mann–Whitney U test according to their distribution.
For univariate analysis, the results were presented as odds ratios (ORs), 95% confidence intervals (CIs) and P values. Significant variables with P value of < 0.1 were then selected into a logistic regression model for multivariate analysis to evaluate risk factors for CRKP BSIs and for K. pneumoniae BSI-related mortality. The discrimination ability of the logistic regression model was assessed by estimating the area under the receiver operating characteristic (ROC) curve. Calibration of the model was assessed using the Hosmer-Lemeshow test for goodness of fit. Two-tailed P value of < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS 17.0 software (IBM Corporation).
Results
Characteristics of patients
We identified a total of 161 unique cases of bloodstream infections with K. pneumoniae during the study period. After excluding 23 cases (polymicrobial BSIs [n = 14]; incomplete medical records [n = 9]), 138 patients with K. pneumoniae BSIs fulfilled the inclusion criteria and were enrolled in this study. Fifty-four cases (39.1%) were identified as CRKP, and 84 (60.9%) were identified as CSKP.
The median patient age was 24.8 months (range, 0 to 204.3 months; interquartile range [IQR], 1.1 to 101.6 months). Patients in the CSKP group were younger than those in CRKP group (10.9 vs. 46.3 months, P = 0.021). Eighty (58.0%) were male, and 58 (42.0%) were female. Most of the BSI (114; 82.6%) were healthcare-associated, while the rest (24; 17.4%) were community-acquired. Most children (118; 85.5%) had at least one underlying disease, including hematologic malignancies (66; 55.9%), congenital anomalies (19; 16.1%), premature at birth (13; 11.0%), solid tumours (8; 6.8%), malnutrition (7; 5.9%), and immunodeficiency (6; 5.1%). One hundred and one patients (73.2%) had a hospitalization history within 90 days prior to the onset of BSIs, and the majority of these (69; 68.3%) had been admitted to hematology-oncology department. The median duration of hospitalization was 21 days (IQR, 15 to 32 days), and the median duration of hospital stay before the onset of BSI was 10 days (IQR, 1 to 15 days). The demographic and clinical data of patients with K. pneumoniae BSI according to CSKP and CRKP are shown in Table 1.
Microbiological characteristics of K. pneumoniae strains
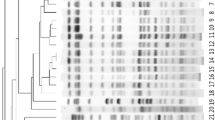
Fifty-four isolates were identified as CRKP. Fifty-one isolates had a meropenem/imipenem MIC ≥ 4 μg/mL. Most of them (40/51, 78.4%) were tested for carbapenemase. The most frequently detected carbapenemase was NDM-1 (23/40, 57.5%) followed by IMP-4 (13/40, 32.5%) and KPC-2 (4/40, 10.0%). Three isolates that were susceptible to meropenem (MIC ≤ 1 μg/mL) and intermediate to imipenem (MIC = 2 μg/mL) were blaIMP-4 harboring. As shown in Table 2, CRKP isolates showed higher rates of resistance than did CSKP isolates. In the CRKP group, resistance to imipenem and meropenem was 94.4 and 85.2%, respectively. The most active drugs were amikacin (susceptibility of 90.7%, 49/54) and ciprofloxacin (susceptibility of 90.7%, 49/54). MIC of meropenem > 8 μg/ml was associated with higher mortality compared with MIC ≤ 1 μg/ml (Fig. 1).
Risk factors for CRKP BSI
To identify risk factors associated with CRKP BSI, we conducted a retrospective case-control study. On univariate analysis, the following factors were most associated with the development of CRKP BSI: age, underlying disease, hematologic malignancies, number of previous hospitalizations, prior presence of intravascular catheter, previous immunosuppressive therapy, previous neutropenia, previous antibiotic therapy, and number of antibiotic agents. Prior exposure to cephalosporin, antifungal agents and glycopeptides were also significant risk factors.
The results of the multivariate analysis are shown in Table 3: the independent risk factors for CRKP BSI were hematologic malignancies (OR:4.712, [95% CI: 2.181–10.180], P < 0.001) and previous cephalosporin administration (OR: 3.427, [95%CI: 1.513–7.766], P = 0.003). The result of Hosmer-Lemeshow chi-square testing (X2 = 0.588; P = 0.745) was indicative of good calibration. The ROC area under the curve was 0.729, suggesting that the multivariate model had good predictive ability.
Treatment and outcome
Antibiotic treatments and outcomes of patients with K. pneumoniae BSI are shown in Table 1 and Fig. 2. Patients with CRKP BSI were less likely to receive active antibiotic agents as empirical treatment than were patients with CSKP BSI (19/54 vs. 63/84, P < 0.001). Empiric therapy was given to all patients with CRKP BSI: 70.4% (38) received meropenem or imipenem, 18.5% (10) received amikacin, and 16.7% (9) received cephalosporins or β-lactam-β-lactamase inhibitors. Thirty-nine patients received one drug as empiric treatment, while nine received in vitro active drug. Only 27.8% (15) patients received a combination of two or more drugs, while 10 received appropriate empiric therapy. Compared with those of other departments, patients in the hematology-oncology ward received a higher proportion of appropriate empiric therapy (17/39 vs. 2/15, P = 0.056). After detection of CRKP BSI, three patients died before antibiotic susceptibility results were available, one of whom had received active antibiotic.
Antibiotic therapy and outcome for patients with CRKP BSIs. AMK, Amikacin; AMC, Amoxycillin/clavulanic acid; CAZ, Ceftazidime; CIP, Ciprofloxacin; CRO, Ceftriaxone; LVX, Levofloxacin; MEM, Meropenem; MOX, Latamoxef; MXF, Moxifloxacin; PEN, Penicillin; SCF, Cefoperazone/sulbactam; TZP, Piperacillin/tazobactam; ZOX, Ceftizoxme. * Died. *# Patients died before blood culture results were available
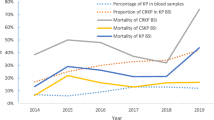
Mortality was significantly higher in patients with CRKP BSI than in those with CSKP BSI (7-day mortality: 16.7% vs. 1.2%, P = 0.001; 28-day mortality: 18.5% vs. 2.4%, P = 0.003). In the CRKP BSI group, 28-day mortality of patients who received at least one active antibiotic agent as empirical treatment was not significantly different from the mortality of patients who did not receive active antibiotic agent (4/19 vs. 6/35, P = 0.728). Carbapenem-including treatment was administered to 39 patients, and their mortality was similar to that of patients who did not receive carbapenem-including treatment (7/39 vs. 3/15, P = 1.0). Overall, 28-day mortality was lower among patients in the hematology-oncology ward (6/39, 15.4%) than in patients in other wards (4/15, 26.7%, P = 0.438).
Univariate analysis indicated that organ dysfunction, septic shock, mechanical ventilation and isolation of CRKP were associated with 28-day mortality. On multivariate analysis, the independent risk factors for 28-day mortality were mechanical ventilation (OR:9.502, [95% CI: 2.098–43.033], P = 0.003), septic shock (OR:6.418, [95% CI: 1.342–30.686], P = 0.020), and isolation of CRKP (OR:9.171, [95% CI: 1.546–54.416], P = 0.015) (Table 4). The area under the ROC curve for this model was 0.878, and the Hosmer-Lemeshow goodness-of-fit P-value was 0.346.
Discussion
CRKP infections are becoming a serious problem in children and are of great concern because of the limited treatment options and unfavourable impact on prognosis [14, 22]. According to previous studies, children with risk factors appear to be more vulnerable to CRKP infections [14]. In the present study, we described the clinical characteristics, risk factors and outcome of BSI due to K. pneumoniae in the paediatric population.
In our study, the predominant carbapenemase was NDM-1. In China, the main factor causing carbapenem resistance was KPC-2 among adults [23]; however, NDM-1- producing K. pneumoniae was most common in children [21]. Three isolates that were susceptible to meropenem and intermediate to imipenem were blaIMP-4 harboring, suggesting that some CRKP may test as susceptible or intermediate to carbapenems [14]. Patients with CRKP BSI were older than those with CSKP; this may be because the majority of them came from the hematology-oncology ward.
Previous studies have identified several risk factors associated with development of CRKP BSI, including exposure to healthcare, previous ICU stay or admission, presence of indwelling devices and exposure to antibiotics (such as cephalosporins, fluoroquinolones and carbapenems) [14,15,16]. We showed that CRKP BSI was associated with several factors including age, underlying disease, number of previous hospitalizations, prior presence of intravascular catheter, previous immunosuppressive therapy, and previous antibiotic therapy. However, only hematologic malignancies and previous cephalosporin administration were independent risk factors for CRKP BSI. Patients with hematologic malignancies usually undergo more frequent exposure to healthcare, longer duration of antibiotic therapy, more invasive procedures and have pre-existing immunosuppression. All of these factors can increase the risk for infections [10, 24]. According to Kwak et al., prior use of carbapenem and cephalosporin were risk factors for acquisition of CRKP [25]. Liu et al. and Orsi et al. found that previous cephalosporin exposure was an independent risk factor ertapenem-resistant K. pneumoniae infections [26, 27]. Our study also suggested that previous cephalosporin administration was associated with CRKP BSI. The carbapenems, fluoroquinolones and glycopeptides were independent risk factors for CRKP infections, according to the previous studies [16, 25, 28]; however, the present study did not show an association between these agents and the development of CRKP BSI.
The overall hospital mortality was 12.3%, lower than that reported in previous paediatric studies [29, 30]. The 28-day mortality in the CRKP group (18.5%) was also lower than that of previous reports, where mortality for CRKP BSI ranged from 39 to 82% [31]. Most recently, Montagnani et al. reported that 4 of 9 children (44.4%) in Italy died from CRKP BSI [22]. Another study demonstrated that the rate of carbapenem-resistant Enterobacteriaceae BSI-related mortality in the Indian paediatric population was 52% [32]. Previous studies suggested that ICU admission was an independent risk factor for death [6, 10]; however, compared with this Indian study (38% were in ICU), there were only 9.3% in our cohort. Patients with hematologic malignancies have relatively better baseline condition and undergo less invasive procedures compare with these who admitted to the ICU. All the above factors were the main predictors of CRKP infection mortality [32]. We supposed that these reasons may explain our low mortality rate. On the other hand, 72.5% patients with CRKP BSI had hematologic malignancies in our cohort. Patients with hematologic malignancies often received more effective empirical treatment due to high clinical suspicion for multidrug-resistant gram-negative bacteria in our hematology-oncology ward. It has been shown that appropriate antimicrobial treatment can help to improve the survival rate [32, 33]. Our study also demonstrated that 28-day mortality was lower among patients in the hematology-oncology ward than in patients in other wards.
Consistently with previous studies [10, 33, 34], the mortality appeared to be higher in CRKP BSI than in CSKP BSI (18.5% vs. 2.4%) in our study, and isolation of CRKP was the independent risk factor for 28-day mortality. A review also suggested that the isolation of CRKP was the main risk factor for mortality from BSI [35]. This finding could be explained by the limited CRKP treatment options. Several studies also indicated that empirical therapy with non-active antibiotics may contribute to unfavourable outcomes [36, 37]. In our study, both the proportions of active empirical and definitive antibiotic treatment were significantly lower in patients with CRKP BSI. However, we did not identify an association between active antibiotic agents and mortality. This may be explained by the small number of cases in this cohort.
We also found mechanical ventilation and septic shock were associated with higher 28-day mortality, consistent with the findings of previous studies [38, 39]. Several reports suggested that age and the seriousness of patients’ conditions (including septic shock) were independent risk factors for mortality [16, 31]. Villegas and colleagues also found that critical illness was a statistically significant factor associated with mortality among patients with BSI caused by carbapenemase-producing Enterobacteriaceae [40]. Xu et al. conducted a meta-analysis of mortality of patients infected with K. pneumoniae and concluded that patients’ physical condition had a close relationship with their survival [10].
There were some limitations in this study. Firstly, it was a retrospective study and was conducted in a single centre, including 138 paediatric patients. This may have influenced the power of the analysis to identify risk factors. Further prospective, multicentre studies are needed. Secondly, we did not test all the CRKP isolates to determine the carbapenem resistance mechanisms; therefore, it is possible for us to overestimate or underestimate the prevalence of NDM-1.
Conclusion
Hematologic malignancies and previous cephalosporin administration were associated with the development of CRKP BSI. We also found low mortality caused by K. pneumoniae BSI in children. Isolation of CRKP was the independent risk factor for mortality, while patients with serious baseline conditions (including septic shock) had higher mortality. Thus, more attention should be paid to CRKP BSI in the paediatric population, especially in patients with a poor state of health.
Abbreviations
- ANC:
-
Absolute neutrophil count
- BSI:
-
Bloodstream infection
- CA:
-
Healthcare-related (HCR) or community-acquired
- CRKP:
-
Carbapenem-resistant K. pneumoniae
- CSKP:
-
Carbapenem-susceptible K. pneumoniae
- ICU:
-
Intensive care unit
- MIC:
-
Minimal inhibition concentration
References
Chen S, Feng W, Chen J, Wei L, He N, Wang Q, Sun F, Xia P. Spread of Carbapenemase-producing Enterobacteria in a southwest Hospital in China. Ann Clin Microb Anti. 2014;13(1):42.
Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602.
Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of Carbapenem-ResistantKlebsiella pneumoniaeInfection and the impact of antimicrobial and adjunctive therapies. Infection Control & Hospital Epidemiology. 2008;29(12):1099–106.
Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli AY. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrobial Agents & Chemotherapy. 2008;52(3):1028.
Falagas ME, Rafailidis PI, Kofteridis D, Virtzili S, Chelvatzoglou FC, Papaioannou V, Maraki S, Samonis G, Michalopoulos A. Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: a matched case control study. J Antimicrob Chemoth. 2007;60(5):1124.
Borer A, Saidelodes L, Riesenberg K, Eskira S, Peled N, Nativ R. Attributable mortality rate for Carbapenem-resistant Klebsiella pneumoniae bacteremia. Infection Control & Hospital Epidemiology. 2009;30(10):972–6.
Hu FP, Guo Y, Zhu DM, Wang F, Jiang XF, Xu YC, Zhang XJ, Zhang CX, Ji P, Xie Y. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clinical Microbiology & Infection the Official Publication of the European Society of Clinical Microbiology & Infectious Diseases 2016, 22 Suppl 1:S9.
Daikos GL, Markogiannakis A, Souli M, Tzouvelekis LS. Bloodstream infections caused by carbapenemase-producing Klebsiella pneumoniae: a clinical perspective. Expert Rev Anti-Infect Ther. 2012;10(12):1393–404.
Broberg CA, Palacios M, Miller VL. Klebsiella: a long way to go towards understanding this enigmatic jet-setter. F1000Prime Rep. 2014;6:64.
Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18.
Bowers DR, Huang V. Emerging issues and treatment strategies in Carbapenem-resistant Enterobacteriaceae (CRE). Curr Infect Dis Rep. 2016;18(12):48.
Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment options for Carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2(2):v50.
Maltezou HC, Kontopidou F, Katerelos P, Daikos G, Roilides E, Theodoridou M. Infections caused by carbapenem-resistant gram-negative pathogens in hospitalized children. Pediatr Infect Dis J. 2013;32(4):151–4.
Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis. 2012;55(6):852.
Tian L, Tan R, Chen Y, Sun J, Liu J, Qu H, Wang X. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5:48.
Jiao Y, Qin Y, Liu J, Li Q, Dong Y, Shang Y, Huang Y, Liu R. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Glob Health. 2015;109(2):68–74.
Hussein K, Raz-Pasteur A, Finkelstein R, Neuberger A, Shachor-Meyouhas Y, Oren I, Kassis I. Impact of carbapenem resistance on the outcome of patients' hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect. 2013;83(4):307–13.
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-e93.
Navarro-San FC, Mora-Rillo M, Romero-Gomez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gomez-Gil R, Arribas-Lopez JR, Mingorance J, Pano-Pardo JR. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect. 2013;19(2):E72–9.
CLSI. Performance standards for antimicrobial susceptibility testing; 24th informational supplement, M100-S24. PA: Wayne; 2014.
Wang S, SUN J, SU W, HU Z, YANG J, Wang Y. Epidemiological analysis of NDM-1-positive bacteria in China. Military Medical Sciences. 2015;39(11):825–30.
Montagnani C, Prato M, Scolfaro C, Colombo S, Esposito S, Tagliabue C, Lo VA, Bruzzese E, Loy A, Cursi L, et al. Carbapenem-resistant Enterobacteriaceae infections in children: an Italian retrospective multicenter study. Pediatr Infect Dis J. 2016;35(8):862–8.
Zheng B, Dai Y, Liu Y, Shi W, Dai E, Han Y, Zheng D, Yu Y, Li M. Molecular epidemiology and risk factors of Carbapenem-resistant Klebsiella pneumoniae infections in eastern China. Front Microbiol. 2017;8
Micozzi A, Gentile G, Minotti C, Cartoni C, Capria S, Ballaro D, Santilli S, Pacetti E, Grammatico S, Bucaneve G, et al. Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae bacteremias. BMC Infect Dis. 2017;17(1):203.
Kwak YG, Choi SH, Choo EJ, Chung JW, Jeong JY, Kim NJ, Woo JH, Ryu J, Kim YS. Risk factors for the acquisition of carbapenem-resistant Klebsiella pneumoniae among hospitalized patients. Microb Drug Resist. 2005;11(2):165–9.
Orsi GB, Garcia-Fernandez A, Giordano A, Venditti C, Bencardino A, Gianfreda R, Falcone M, Carattoli A, Venditti M. Risk factors and clinical significance of ertapenem-resistant Klebsiella pneumoniae in hospitalised patients. J Hosp Infect. 2011;78(1):54–8.
Liu SW, Chang HJ, Chia JH, Kuo AJ, Wu TL, Lee MH. Outcomes and characteristics of ertapenem-nonsusceptible Klebsiella pneumoniae bacteremia at a university hospital in northern Taiwan: a matched case-control study. J Microbiol Immunol Infect. 2012;45(2):113–9.
Gomez RV, Zuleta TJ. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae: a case-case-control study. Colomb Med (Cali). 2014;45(2):54–60.
Zaoutis TE, Goyal M, Chu JH, Coffin SE, Bell LM, Nachamkin I, McGowan KL, Bilker WB, Lautenbach E. Risk factors for and outcomes of bloodstream infection caused by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species in children. Pediatrics. 2005;115(4):942–9.
Kim YK, Pai H, Lee HJ, Park SE, Choi EH, Kim J, Kim JH, Kim EC. Bloodstream infections by extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother. 2002;46(5):1481–91.
de Maio CC, de Oliveira LM, Gaudereto J, Perozin JS, Urbano MR, Camargo CH, Grion CM, Levin AS, Costa SF. A prospective study of treatment of carbapenem-resistant Enterobacteriaceae infections and risk factors associated with outcome. BMC Infect Dis. 2016;16(1):629.
Nabarro LE, Shankar C, Prakasam A, Mathew G, Jeyaseelan V, Veeraraghavan B, Verghese VP. Clinical and bacterial risk factors for mortality in children with Carbapenem- resistant Enterobacteriaceae bloodstream infections in India. Pediatr Infect Dis J. 2017;36(6):e161-e166.
Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17(12):1798–803.
Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del BV, Corcione S, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70(7):2133–43.
Viale P, Giannella M, Lewis R, Trecarichi EM, Petrosillo N, Tumbarello M. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev Anti-Infect Ther. 2013;11(10):1053–63.
Akturk H, Sutcu M, Somer A, Aydin D, Cihan R, Ozdemir A, Coban A, Ince Z, Citak A, Salman N. Carbapenem-resistant Klebsiella pneumoniae colonization in pediatric and neonatal intensive care units: risk factors for progression to infection. Braz J Infect Dis. 2016;20(2):134–40.
Satlin MJ, Cohen N, Ma KC, Gedrimaite Z, Soave R, Askin G, Chen L, Kreiswirth BN, Walsh TJ, Seo SK. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J Inf Secur. 2016;73(4):336–45.
Mariappan S, Sekar U, Kamalanathan A. Carbapenemase-producing Enterobacteriaceae: risk factors for infection and impact of resistance on outcomes. Int J Appl Basic Med Res. 2017;7(1):32–9.
Chang YY, Chuang YC, Siu LK, Wu TL, Lin JC, Lu PL, Wang JT, Wang LS, Lin YT, Huang LJ, et al. Clinical features of patients with carbapenem nonsusceptible Klebsiella pneumoniae and Escherichia coli in intensive care units: a nationwide multicenter study in Taiwan. J Microbiol Immunol Infect. 2015;48(2):219–25.
Villegas MV, Pallares CJ, Escandon-Vargas K, Hernandez-Gomez C, Correa A, Alvarez C, Rosso F, Matta L, Luna C, Zurita J, et al. Characterization and clinical impact of bloodstream infection caused by Carbapenemase-producing Enterobacteriaceae in seven Latin American countries. PLoS One. 2016;11(4):e154092.
Acknowledgements
The abstract has been previously published as a conference abstract and accepted as the poster presentation in 10th World Congress of the World Society for Pediatric Infectious Diseases (WSPID 2017) Session “Clinical Infectious Disease” Shenzhen, China in December 2- December 5, 2017.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
All authors contributed to this work. All authors read and approved the final manuscript. GL, FD, LYG, YW and WQS designed the study. YZ collected the data. GL, FD and YZ interpreted the data. YZ wrote the first draft of the paper. GL, FD and LYG reviewed and approved the final report.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Committee of Beijing Children’s Hospital Affiliated to Capital Medical University (2017-k-83). Informed consent was waived because this was a retrospectively study. We obtained patient data from the Medical Records and Statistics Room. We analysed the data anonymously.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, Y., Guo, LY., Song, WQ. et al. Risk factors for carbapenem-resistant K. pneumoniae bloodstream infection and predictors of mortality in Chinese paediatric patients. BMC Infect Dis 18, 248 (2018). https://doi.org/10.1186/s12879-018-3160-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-018-3160-3