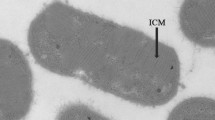
Abstract
A new obligately methylotrophic bacterium, strain OVT, was isolated from roots of sedge (Carex sp.). The isolate was represented by aerobic gram-negative motile, non-spore-forming rods, which divided by binary fission. Optimal growth occurred at 22−29°C and pH 7.5−8.5 in the presence of 0.5−2% NaCl; growth was inhibited by 3.5% NaCl. Strain OVT utilized methanol as the only carbon and energy source. The organism used the KDPG variant of the ribulose monophosphate pathway (RuMP) of С1 metabolism. Ammonium was assimilated by reductive amination of α-ketoglutarate. The major cellular fatty acids were C16:0 (45.5%), C16:1ω7c (40.7%), and C17cyc (8.0%). The major ubiquinone was Q8. The dominant phospholipids were phosphatidylethanolamine, phosphatidylglycerol, and diphosphatidylglycerol. The DNA G + C content of strain OVT was 51.4 mol % (Tm). While the 16S rRNA gene sequence of strain OVT exhibited high similarity to those of Methylobacillus species: M. gramineus LapT (99.6%) and M. glycogenes TK 0113T (98.7%), the DNA-DNA hybridization level between strain OVT and M. gramineus LapT was only 52%. Based on the data obtained, strain OVT was assigned to the new species Methylobacillus caricis sp. nov. (=VKM B-3158T = JCM 32031T).
Similar content being viewed by others
References
Balachandar, D., Raja, P., and Sundaram, S.P., Genetic and metabolic diversity of pink-pigmented facultative methylotrophs in phyllosphere of tropical plants, Braz. J. Microbiol., 2008, vol. 39, pp. 68–73.
Collins, M.D., Analysis of isoprenoid quinones, in Methods in Microbiology, Gottschalk, G., Ed., New York: Academic, 1985, vol. 18, pp. 329–366.
Doronina, N.V., Gogleva, A.A., and Trotsenko, Y.A., Methylophilus glucosoxydans sp. nov., a restricted facultative methylotroph from rice rhizosphere, Int. J. Syst. Evol. Microbiol., 2012, vol. 62, pp. 196–201.
Doronina, N.V., Govorukhina, N.I., Lysenko, A.M., and Trotsenko, Y.A., Analysis of DNA–DNA homology in obligately methylotrophic bacteria, Microbiology (Moscow), 1988, vol. 57, pp. 509–513.
Doronina, N.V., Trotsenko, Y.A., Kolganova, T.V., Tourova, T.P., and Salkinoja-Salonen, M.S., Methylobacillus pratensis sp. nov., a novel non-pigmented, aerobic, obligately methylotrophic bacterium isolated from meadow grass, Int. J. Syst. Evol. Microbiol., 2004, vol. 54, pp. 1453–1457.
Gogleva, A.A., Kaparullina, E.N., Doronina, N.V., and Trotsenko, Y.A., Methylobacillus arboreus sp. nov. and Methylobacillus gramineus sp. nov., novel non-pigmented obligately methylotrophic bacteria associated with plants, Syst. Appl. Microbiol., 2011, vol. 34, pp. 477–481.
Gordon, S.A. and Weber, R.P., Colorimetric estimation of indoleacetic acid, Plant Physiol., 1951, vol. 26, pp. 192–195.
Govorukhina, N.I., Kletsova, L.V., Tsygankov, Yu.D., Trotsenko, Yu.A., and Netrusov, A.I., Characteristics of a new obligate methylotroph, Microbiology (Moscow), 1987, vol. 56, pp. 673–678.
Kaparullina, E.N., Doronina, N.V., Mustakhimov, I.I., Agafonova, N.V., and Trotsenko, Yu.A., Biodiversity of aerobic methylobacteria associated with the phyllosphere of the southern Moscow region, Microbiology (Moscow), 2017, vol. 86, no. 1, pp. 113–118.
Kaparullina, E.N., Trotsenko, Yu.A., and Doronina, N.V., Methylobacillus methanolivorans sp. nov., a novel non-pigmented obligately methylotrophic bacterium, Int. J. Syst. Evol. Microbiol., 2017, vol. 67, pp. 425–431.
Lane, D.J., 16S/23S rRNA sequencing, in Nucleic Acid Techniques in Bacterial Systematics, Stackebrandt, E. and Goodfellow, M., Eds., Chichester: Wiley, 1991, pp. 115–175.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J., Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, vol. 193, pp. 265–275.
Madhaiyan, M., Poonguzhali, S., Senthilkumar, M., Pragatheswari, D., Lee, K.-C., and Lee, J.-S., Methylobacillus rhizosphaerae sp. nov., a novel plant-associated methylotrophic bacterium isolated from rhizosphere of red pepper, Antonie van Leeuwenhoek, 2013, vol. 103, pp. 475–484.
McDonald, I.R. and Murrell, J.C., The methanol dehydrogenase structural gene mxaF and its use as a functional gene probe for methanotrophs and methylotrophs, Appl. Environ. Microbiol., 1997, vol. 63, pp. 3218–3224.
Owen, R.J. and Lapage, S.P., The thermal denaturation of partly purified bacterial deoxyribonucleic acid and its taxonomic applications, J. Appl. Bacteriol., 1976, vol. 41, pp. 335–340.
Saitou, N. and Nei, M., The neighbour-joining method: a new method for reconstructing phylogenetic trees, Mol. Biol. Evol., 1987, vol. 4, pp. 405–425.
Sasser, M., Identification of bacteria by gas chromatography of fatty acids, MIDI Technical Note 101, Newark, DE: MIDI, Inc., 1990.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S., MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods, Mol. Biol. Evol., 2011, vol. 28, pp. 2731–2739.
Trotsenko, Y.A., Doronina, N.V., and Govorukhina, N.I., Metabolism of non-motile obligately methylotrophic bacteria, FEMS Microbiol. Lett., 1986, vol. 3, pp. 293–297.
Trotsenko, Yu.A., Doronina, N.V., and Torgonskaya, N.V., Aerobnye metilobakterii (Aerobic Methylobacteria), Pushchino: ONTI PNTs RAN, 2010.
Urakami, T. and Komagata, K., Emendation of Methylobacillus Yordy and Weaver 1977, a genus of methanol-utilizing bacteria, Int. J. Syst. Bacteriol., 1986, vol. 36, pp. 502–511.
Wellner, S., Lodders, N., Glaeser, S.P., and Kämpfer, P., Methylobacterium trifolii sp. nov. and Methylobacterium thuringiense sp. nov., methanol-utilizing, pink-pigmented bacteria isolated from leaf surfaces, Int. J. Syst. Evol. Microbiol., 2013, vol. 63, pp. 2690–2699.
Yordy, J.R. and Weaver, T.L., Methylobacillus: a new genus of obligately methylotrophic bacteria, Int. J. Syst. Bacteriol., 1977, vol. 27, pp. 247–255.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.V. Agafonova, E.N. Kaparullina, N.V. Doronina, Yu.A. Trotsenko, 2017, published in Mikrobiologiya, 2017, Vol. 86, No. 6, pp. 720–728.
Rights and permissions
About this article
Cite this article
Agafonova, N.V., Kaparullina, E.N., Doronina, N.V. et al. Methylobacillus caricis sp. nov., an obligate methylotroph isolated from roots of sedge (Carex sp.). Microbiology 86, 737–744 (2017). https://doi.org/10.1134/S0026261717060042
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261717060042