Abstract
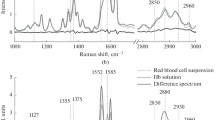
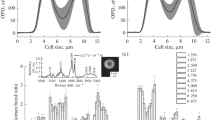
The variation in the conformations of hemoglobin heme and globin in response to different oxygen partial pressures (pO2) has been studied by Raman spectroscopy inside erythrocytes and in hemoglobin dissolved in incubation medium. The spectra of hemoglobin inside erythrocytes and in the medium show that several characteristic bands in the range from 1000 to 1700 cm–1 (stretching vibrations in the heme group) and in the high-frequency range from 2800 to 3000 cm–1 (stretching vibrations in globin amino acids) vary with pO2 levels. The curve of hemoglobin saturation with oxygen (sO2) obtained from the Raman-scattering data displays sigmoidal behavior only in hemoglobin inside erythrocytes. It has been shown that differences in heme conformation at different pO2 levels affect the ability of hemoglobin to form complexes with nitric oxide. As the pO2 level increases, the conformational changes of hemoglobin heme in the cell entail an increase in the contribution of vibrations of lateral –CH3 groups in pyrrole half-rings and group vibrations of bonds inside pyrrole half-rings, whereas the globin conformation is associated with a higher contribution of vibrations from H-methine groups and symmetrical terminal methylene groups in amino acids.



Similar content being viewed by others
REFERENCES
P. Hilpert, R. G. Fleischmann, D. Kempe, et al., Am. J. Physiol. 205 (2), 337 (1963).
M. Berenbrink, Respir. Physiol. Neurobiol. 154 (1–2), 165 (2006).
B. F. Verigo, Arch. Gesamte Physiol Menschen Tiere 51, 321 (1892).
Ch. Bohr, K. Hasselbalch, and A. Krog, Skand. Arch. Physiol. 16, 401 (1904).
M. F. Perutz, Annu. Rev. Biochem. 48, 327 (1979).
F. B. Jensen, Acta Physiol. Scand. 182 (3), 215 (2004).
M. I. Nikinmaa, Physiol. Rev. 72 (2), 301 (1992).
B. Berenbrink, M. Berenbrink, and Ch. Bridges, J. Exp. Biol. 192 (1), 253 (1994).
M. Berenbrink, P. Koldkjar, O. Kepp, et al., Science 307 (5716), 1752 (2005).
R. Motais, F. Garcia-Romeu, and F. Borgese, J. Gen. Physiol. 90 (2), 197 (1987).
P. Giardina, V. Aurilia, R. Cannio, et al., Eur. J. Biochem. 235 (3), 508 (1996).
N. Barvitenko, N. Adragna, and R. Weber, Cell. Physiol. Biochem. 15 (1–4), 1 (2005).
O. V. Slatinskaya, O. G. Luneva, and L. I. Deev, Biophysics 65 (2), 213 (2020).
14. J. T. Edsall, J. Hist. Biol. 5 (2), 205 (1972).
B. Wranne, R. D. Woodson, and J. C. Detter, J. Appl. Physiol. 32 (6), 749 (1972).
J. B. West, Am. J. Physiol. – Lung Cell. Mol. Physiol. 316 (4), 585, (2019).
C. Bohr and K. Hasselbalch, Skand. Arch. Physiol. 16, 402 (1904).
S. V. Sidorenko, R. H. Ziganshin, O. G. Luneva, et al., J. Proteomics 184, 25 (2018).
N. A. Brazhe, S. Abdali, A. R. Brazhe, et al., Biophys. J. 97 (12), 3206 (2009).
S. C. Goheen, L. J. Lis, O. Kucuk, et al., J. Raman Spectrosc. 24 (9), 275 (1993).
I. P. Torres Filho, J. Terner, R. N. Pittman, et al., J, Appl. Physiol. 104 (6), 1809 (2008).
B. R. Wood, L. Hammer, and D. McNaughton, Vibr. Spectrosc. 38 (1–2), 78 (2005).
O. G. Luneva, S. V. Sidorenko, O. O. Ponomarchuk, et al., Cell. Physiol. Biochem. 39 (1), 81 (2016).
B. R. Wood, P. Caspers, G. J. Puppels, et al., Anal. Bioanal. Chem. 387 (5), 1691 (2007).
I. P. Torres Filho, J. Terner, R. N. Pittman, et al., Am. J. Physiol. – Heart Circ. Physiol. 289, 488 (2005).
G. Chottard and D. Mansuy, Biochem. Biophys. Res. Commun. 77 (4), 1333 (1977).
A. Szabo and L. D. Barron, J. Am. Chem. Soc. 97 (3), 660 (1975).
G. Louie, T. Tran, J. I. Englander, and S. W. Englander, Mol. BioI. 201, 755 (1988).
Y. Sun, A. Benabbas, W. Zeng, et al. Proc. Natl Acad. Sci. U. S. A. 111 (18), 6570 (2014).
R. V. Chertkova, N. A. Brazhe, T. V. Bryantseva, et al., PLoS One 12 (5), e0178280 (2017).
R. Liddington, Z. Derewenda, G. Dodson, and D. Harris Nature 331, 725 (1988).
A. Klug, F. Kreuzer, and F. J. W. Roughton, Physiol. Acta 14, 121 (1956).
R. Zander and H. Schmid-Schoenbien, Respir. Physiol. 19, 279 (1973).
K. D. Vandegriff and J. S. Olson, J. Biol. Chem. 259, 12609 (1984).
S. T. Bouwer, L. Hoofd, and F. Kreuzer, Biochim. Biophys. Acta 1338, 127 (1997).
Funding
O.V.S. acknowledges The reported study was funded by RFBR, project no. 20-34-90073. G.V.M. acknowledges the support of the Russian Science Foundation, project 19-7930062. This work was also supported by the Interdisciplinary research and educational school of the Moscow State University, Molecular technologies of living systems and synthetic biology project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare no conflicts of interests.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the WMA International Code of Medical Ethics and were approved by the Ethical Committee of the Department of Biology, Lomonosov Moscow State University.
Additional information
Translated by V. Gulevich
Abbreviations: Hb, hemoglobin; AE1, band 3, anion exchanger 1; dHb, deoxyhemoglobin; oHb, oxyhemoglobin.
Rights and permissions
About this article
Cite this article
Slatinskaya, O.V., Luneva, O.G., Deev, L.I. et al. The Hemoglobin Conformation in Erythrocytes at Different Levels of Oxygen Partial Pressure. BIOPHYSICS 66, 797–803 (2021). https://doi.org/10.1134/S0006350921050225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350921050225