Abstract
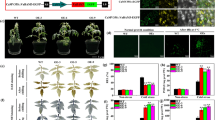
Chlorophyll reduction in the seed of Brassica can be achieved by downregulating its synthesis. To reduce chlorophyll synthesis, we have used a cDNA clone of Brassica napus encoding glutamate 1-semialdehyde aminotransferase (GSA-AT) to make an antisense construct for gene manipulation. Antisense glutamate 1-semialdehyde aminotransferase gene (Gsa) expression, directed by a Brassica napin promoter, was targeted specifically to the embryo of the developing seed. Transformants expressing antisense Gsa showed varying degrees of inhibition resulting in a range of chlorophyll reduction in the seeds. Seed growth and development were not affected by reduction of chlorophyll. Seeds from selfed transgenic plants germinated with high efficiency and growth of seedlings was vigorous. Seedlings from T2 transgenic lines segregated into three distinctive phenotypes: dark green, light green and yellow, indicating the dominant inheritance of Gsa antisense gene. These transgenic lines have provided useful materials for the development of a low chlorophyll seed variety of B. napus.
Similar content being viewed by others
References
AOCS Official Method Am 2-93 1998. Determination of oil content in oilseeds. Official Methods & Recommended Practices of the AOCS 5th Edn. Am Oil Chem Soc, Champaign, Ill., pp. 1-5.
AOCS Official Method Ba 4e-93 1998. Generic combustion method for determination of crude protein. Official Methods & Recom-mended Practices of the AOCS 5th Edn. Am Oil Chem Soc, Champaign, Ill., pp. 1-4.
Arnon, D.I. 1964. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24: 1-5.
Beffa, R.S., Neuhaus, J-M. and Meins Jr, F. 1993. Physiological compensation in antisense transformants: specific induction of an ‘ersatz’ glucan endo-1,3-â-glucosidase in plants infected with necrotizing viruses. Proc. Natl. Acad. Sci. USA 90: 8792-8796.
Bowler, C., Neuhaus, G., Yamagata, H. and Chua, N.H. 1994. Cyclic GMP and calcium mediate phytochrome phototransduc-tion. Cell77: 73-81.
Cannon, M., Platz, J., O'Leary, M., Sookdeo, C. and Can-non, F. 1990. Organ-specific modulation of gene expression in transgenic plants using antisense RNA. Plant Mol. Biol. 15: 39-47.
Eastmond, P.J. and Rawsthorne, S. 1998. Comparison of the metabolic properties of plastids isolated from developing leaves or embryos of Brassica napus L. J. Expt. Bot. 49: 1105-1111.
Fling, S.P. and Gregerson, D.S. 1986. Peptide and protein molecular weight determination by electrophoresis using a high molarity Tris buffer system without urea. Anal. Biochem. 155: 83-88.
Hamilton, A.L., Lycett, G.W. and Grierson, D. 1990. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 346: 284-287.
Höfgen, R., Axelsen, K.B., Kannangara, C.G., Schüttke, I., Pohlenz, H.D., Willmitzer, L., Grimm, B. and von Wettstein, D. 1994. A visible marker for antisense mRNA expression in plants: inhibition of chlorophyll synthesis with a glutamate-1-semialdehyde aminotransferase antisense gene. Proc. Natl. Acad. Sci. USA 91: 1726-1730.
Huang, D.D., Wang, W.Y., Gough, S.P. and Kannangara, C.G. 1984. ä-aminolevulinic acid-synthesing enzymes need an RNA moiety for activity. Science 225: 1482-1484.
Hughes, J. and Lamparter, T. 1999. Prokaryotes and phytochrome: the connection to chromophores and signaling. Plant Physiol. 121: 1059-1068.
Ilag, L.L., Kumar, A.M. and Söll, D. 1994. Light regulation of chlorophyll biosynthesis at the level of 5-aminolevulinate formation in Arabidopsis. Plant Cell 6: 265-275.
Jefferson, R.A. 1988. Assaying chimeric genes in plants: the Gus gene fusion system. Plant Mol. Biol. 5: 389-405.
Jorgensen, R.A., Cluster, P.D., English, J., Que, Q. and Napoli, C.A. 1996. Chalcone synthase cosuppression phenotypes in petunia flowers comparison of sense vs. antisense constructs and single-copy vs. complex T-DNA sequences. Plant Mol. Biol. 31: 957-973.
Kalmokoff, M.L., Pickard, M.D. and GrootWassink, J.W.D. 1988. Resistance of green pigments in commercial canola oil to enzy-matic hydrolysis. Can. Inst. Food Sci. Technol. J. 21: 534-536.
Kannangara, C.G. and Gough, S.P. 1978. Biosynthesis of ä-aminolevulinate in greening barley leaves: glutamate 1-semialdehyde aminotransferase. Carlsberg Res. Commun. 43: 185-194.
Kannangara, C.G., Gough, S.P., Oliver, R.P. and Rasmussen, S.K. 1984. Biosynthesis of ä-aminolevulinate in greening barley leaves VI. Activation of glutamate by ligation to RNA. Carlsberg Res. Commun. 49: 417-437.
Kannangara, C.G., Gough, S.P., Bruyant, P., Hoober, J.K., Kahn, A. and von Wettstein, D. 1988. tRNA Glu as a cofactor in ä-aminolevulinate biosynthesis: steps that regulate chlorophyll synthesis. Trends Biochem. Sci. 13: 139-143.
King, S.P., Badger, M.R. and Furbank, R.T. 1998. CO 2 refixation characteristics of developing canola seeds and silique wall. Aust. J. Plant Physiol. 25: 377-386.
Kimber, D.S. and McGregor, D.I. 1995. The species and their origin, cultivation and world production. Pp. 1-7. In: Kimber, D., Mc-Gregor, D.I. (Eds) Brassica oilseeds production and utilization. Cab International, Wallingford, Oxon, UK.
Kohno-Murase, J., Murase, M., Ichikawa, H. and Imamura, J. 1994. Effects of an antisense napin gene on seed storage compounds in transgenic Brassica napus seeds. Plant Mol. Biol. 26: 1115-1124.
Koncz, C. and Schell, J. 1986. The promoter of T L-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium vector. Mol. Gen. Genet. 204: 383-396.
Levadoux, W.L., Kalmokoff, M.L., Pickard, M.D. and Groot-Wassink, J.W.D. 1987. Pigment removal from canola oil using chlorophyllase. J. Am. Oil Chem. Soc. 64: 139-144.
Matzke, A.J. and Matzke, M.A. 1998. Position effects and epigenetic silencing of plant transgenes. Curr. Opin. Plant Biol. 1: 142-148.
Moloney, M.M., Walker, J.M. and Sharma, K.K. 1989. High ef-ficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep. 8: 238-242.
Nakayashiki, T., Nishimura, K., Tanaka, R. and Inokuchi, H. 1995. Partial inhibiton of protein synthesis accelerates the synthesis of porphyrin in heme-deficient mutants of Escherichia coli. Mol. Gen. Genet. 249: 139-146.
Neuhaus, J-M., Flores, S., Keefe, D., Ahl-Goy, P. and Meins Jr, F. 1992. The function of vacuolar â-1,3-glucanase investigated by antisense transformation. Susceptibility of transgenic Nicotiana sylvestris plants to Cercospora nicotianae infection. Plant Mol. Biol. 19: 803-813.
Oliver, M.L., Ferguson, D.L., Burke, J.J. and Velten, J. 1993. Inhibi-tion of tabacco NADH-hydroxypyruvate reductase by expression of a heterologous antisense RNA derived from a cucumber cDNA: implications for the mechanism of action of antisense RNAs. Mol. Gen. Genet. 239: 425-434.
Porra, R.J., Thompson, W.A. and Kriedemann, P.E. 1989. Deter-mination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chloro-phyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975: 384-394.
Pugh, C.E., Nair, S.P., Harwood, J.L. and John, R.A. 1991. Conditions for the assay of glutamate semialdehyde aminotrans-ferase that overcome the problem of substrate instability. Anal. Biochem. 198: 43-46.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual 2nd Edn. Cold Spring Harbor Lab. Press, Plainview, NY.
Schafer, U.A., Reed, D.W., Hunter, D.G., Yao, K., Weninger, A.M., Tsang, E.W.T., Reaney, M.J.T., Mackenzie, S.L. and Covello, P.S. 1999. An example of intron junctional sliding in the gene families encoding squalene monooxygenase homologues in Arabidopsis thaliana and Brassica napus. Plant Mol. Biol. 39: 721-728.
Sheeby, R.E., Kramer, M. and Hiatt, W.R. 1988. Reduction of poly-galacturonase activity in tomato fruit by antisense RNA. Proc. Natl. Acad. Sci. USA 85: 8805-8809.
Singh, R.D. and Chuaqui, C.A. 1991. Development of a continuous process to remove chlorophylls from canola oil. Ninth Project Report: Research on canola seed oil and meal, pp. 449-472. Canola Council of Canada, Winnepeg, Canada.
Smith, C.J.S., Watson, C.F., Morris, P.C., Bird, C.R., Seymour, G.B., Gray, J.E., Arnold, C., Tucker, G.A., Schuchk, W., Hard-ing, S. and Grierson, D. 1990. Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol. Biol. 14: 369-379(1990).
Temple, S.J., Knight, T.J., Unkefer, P.J. and Sengupta-Gopalan, C. 1993. Modulation of glutamine synthetase gene expression in to-bacco by the introduction of an alfalfa glutamine synthetase gene in sense and antisense: molecular and biochemical analysis. Mol. Gen. Genet. 236: 315-325.
Tieman, D.M., Harriman, R.V., Ramamohan, G. and Handa, A.K. 1992. An antisense pectin methylesterase gene alters pectin chemistry and soluble solids in tomato fruit. The Plant Cell.: 667-679.
Uppström, B. 1995. Seed chemistry. Pp. 217-242. In: Kimber D., McGregor D.I. (Eds) Brassica oilseeds production and utiliza-tion. Cab International, Wallingford, Oxon, UK.
van der Krol, A.R., Lenting, P.E., Veenstra, J., van der Meer, I.M., Koes, R.E., Gerats, A.G.M., Mol, J.N.M., Stuitje, A.R. 1988. An anti-sense chalcone synthase gene in transgenic plants inhibits flower pigmentation. Nature 333: 866-869.
van der Krol, A.R., Muir, L.A., de Lange, P., Mol, J.N.M. and Stu-itje, A.R. 1990. Inhibition of flower pigmentation by antisense CHS genes: promoter and minimal sequence requirements for the antisense effect. Plant Mol. Biol. 14: 457-466.
van Leeuwen, W., Mlynárová, L., Nap, J.P., van der Plas, L.H.W., van der Krol, A.R. 2001. The effect of MAR elements on vari-ation in spatial and temporal regulation of transgene expression. Plant Mol. Biol. 47: 543-554.
Weinstein, J.D., Beale, S.I. 1985. RNA is required for enzymatic conversion of glutamate to ä-aminolevulinate by extracts of Chlorella vulgaris. Arch. Biochem. Biophy. 239: 87-93.
Welters, P., Takegava, K., Emr, S.D. and Chrispeels, M.J. 1994. AtVP34, a phosphatidylinositol 3-kinase of Arabidopsis thaliana, is an essential protein with homology to a calcium-dependent lipid binding domain. Proc. Natl. Acad. Sci. USA 91: 11398-11402.
Whitmarsh, J. 1998. Electron transport and energy transduction. Pp. 87-107. In: Raghavendra, A.S. (Ed) Photosynthesis: A Com-prehensive Treatise Cambridge. University Press, Cambridge, UK.
Zabaleta, E., Oropeza, A., Assad, N., Mandel, A., Salerno, G. and Herrera-Estrella, L. 1994. Antisense expression of chaperonin 60 in transgenic tobacco plants leads to abnormal phenotypes and altered distribution of photoassimilates. Plant J. 6: 425-432.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsang, E.W., Yang, J., Chang, Q. et al. Chlorophyll reduction in the seed of Brassica napus with a glutamate 1-semialdehyde aminotransferase antisense gene* . Plant Mol Biol 51, 191–201 (2003). https://doi.org/10.1023/A:1021102118801
Issue Date:
DOI: https://doi.org/10.1023/A:1021102118801