Abstract
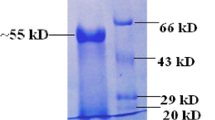
An aqueous two-phase system based on the two polymers poly(ethylene glycol) and dextran has been used for the fractionation of cellulase enzymes present in culture liquid obtained by fermentation with Trichoderma reesei. The activities of β-glucosidase and glucanases were separated to high degree by using the two-phase systems for a counter-current distribution process in nine transfer steps. While the glucanases had high affinity to the poly(ethylene glycol) rich top phase the β-glucosidase was enriched in the dextran-containing bottom phase. Multiple counter-current distribution performed indicates the heterogeneity of β-glucosidase activities assuming at least four isoenzyme forms. One step concentration of β-glucosidase by using system with 46:1 phase volume ratio resulted in 16 times higher enzyme activity.
Similar content being viewed by others
References
Albertsson P-Å (1986) Partition of Cell Particles and Macromolecules, 3rd ed., Wiley, New York.
Albertsson P-Å and Tjerneld F (1994) Phase diagrams. Methods Enzymol. 228: 3–13.
Albertsson P-Å, Andersson B, Larsson C and Åkerlund H-E (1981) Phase partition-a method for purification and analysis of cell organelles and membrane vesicle. Method Biochem. Anal. 28: 115–150.
Bhikhabhai R, Johansson G and Petterson G (1984) Isolation of cellulolytic enzymes from Trichoderma reeseiQM 9414. J. Appl. Biochem. 6: 336.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Chen H, Hayn M and Esterbauer H (1992) Purification and characterization of two extracellular β-glucosidases from Trichoderma reesei. Biochim. Biophys. Acta 1121: 54–60.
Eriksson E and Johansson G (1979) Partition of cells between aqueous phases. In: Reid E (ed.) Methodological Surveys (B) Biochemistry 8 (pp. 81–90). Horwood, Chichester.
Johansson G (1974) Effects of salt on the partitioning of proteins in aqueous polymeric biphasic systems. Acta Chem. Scand. B28: 873–882.
Johansson G and Andersson M (1984) Liquid-liquid extraction of glycolytic enzymes from bakers yeast using triazine dye ligand. J. Chromatogr. 291: 175–183.
Johansson G (1989) Affinity partitioning of proteins using aqueous two-phase systems. In: Janson J-C and Rydén L (eds.) Protein purification, principle, high resolution methods and application (pp. 330–345). VCH Publishers, Inc, New York.
Johansson and Réczey (1998) Concentration and purification of β-glucosidase from Aspergillus nigerby using aqueous two-phase partitioning. J. Chromatogr. B 711: 161–172.
Mandels M, Andreotti R and Rooche C (1976) Measurement of saccharifying cellulase. Biotechnol. Bioeng. Symp. 6: 21–33.
Mandels M and Weber J (1969) The production of cellulases. Adv. Chem. Ser. 95: 391–414.
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analyt. Chem. 31: 426–428.
Morris CJOR and Morris P (1964) Separation Methods in Biochemistry (pp. 559–619). Pitman, London.
Norkrans B (1957) Studies of β-glucosidase and cellulose splitting enzymes from Polyporus annosus Fr. Physiol. Plantarum 10: 198–213.
Reinikainen T (1944) The cellulose-binding domain of cellobi-ohydrolase I from Trichoderma reesei. Interaction with cellulose and application in protein immobilization. Ph.D. Thesis, VTT Technical Research Center of Finland, Espoo.
Sternberg D, Vijayakumar P and Reesei ET (1977) β-Glucosidase: microbial production and effect on enzymatic hydrolysis of cellulose. Can. J. Microbiol. 23: 139–147.
Tjerneld F, Persson I, Albertsson P-Å and Hahn-Hägerdal B (1985a) Enzymatic hydrolysis of cellulose in aqueous two-phase systems. I, Partition of cellulases from Trichoderma reesei. Biotechnol. Bioeng. 27: 1036–1043.
Tjerneld F, Persson I and Albertsson P-Å (1985b) Enzymatic hydrolysis of cellulose in aqueous two-phase systems. II, Semicontinuous conversion of a model substrate, Solka-Floc BW 200. Biotechnol. Bioeng. 27: 1044–1050.
Tjerneld F, Johansson G and Joelsson M (1987) Affinity liquid-liquid extraction of lactate dehydrogenase on a large scale. Biotechnol. Bioeng. 30: 809–816.
Tjerneld F (1992) Aqueous two-phase partitioning on an industrial scale. In: Harris M (ed.) Poly(ethylene glycol) chemistry: Biotechnological and biomedical application (pp. 85–102). Plenum Press, New York.
Walter H and Johansson G (eds.) (1994), Methods Enzymology (vol. 228). Aqueous two-phase systems. Academic Press, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brumbauer, A., Johansson, G. & Réczey, K. Fractionation of cellulase and β-glucosidase in a Trichoderma reesei culture liquid by use of two-phase partitioning. Bioseparation 7, 287–295 (1998). https://doi.org/10.1023/A:1008153418019
Issue Date:
DOI: https://doi.org/10.1023/A:1008153418019