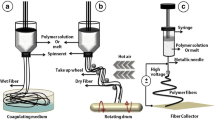
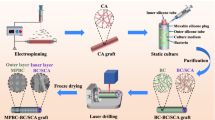
Abstract
The high incidence of cardiovascular disease has led to significant demand for synthetic vascular grafts in clinical applications. Anti-proliferation drugs are usually loaded into devices to achieve desirable anti-thrombosis effects after implantation. However, the non-selectiveness of these conventional drugs can lead to the failure of blood vessel reconstruction, leading to potential complications in the long term. To address this issue, an asymmetric membrane was constructed through electro-spinning techniques. The bilayer membrane loaded and effectively released nitric oxide (NO), as hoped, from only one side. Due to the short diffusion distance of NO, it exerted negligible effects on the other side of the membrane, thus allowing selective regulation of different cells on both sides. The released NO boosted the growth of endothelial cells (ECs) over smooth muscle cells (SMCs)—while on the side where NO was absent, SMCs grew into multilayers. The overall structure resembled a native blood vessel, with confluent ECs as the inner layer and layers of SMCs to support it. In addition, the membrane preserved the normal function of ECs, and at the same time did not exacerbate inflammatory responses. By preparing this material type that regulates cell behavior differentially, we describe a new method for its application in the cardiovascular field such as for artificial blood vessels.
Graphic abstract






Similar content being viewed by others
References
PrimeView (2019) Atherosclerosis. Nat Rev Dis Primers 5(1):57. https://doi.org/10.1038/s41572-019-0116-x
Serruys PW, de Jaegere P, Kiemeneij F et al (1994) A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med 331:489–495. https://doi.org/10.1056/NEJM199408253310801
Ren X, Feng Y, Guo J et al (2015) Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem Soc Rev 44(15):5745. https://doi.org/10.1039/C4CS00483C
Li S, Sengupta D, Chien S (2014) Vascular tissue engineering: from in vitro to in situ. Wiley Interdiscip Rev Syst Biol Med 6(1):61–76. https://doi.org/10.1002/wsbm.1246
Angiolillo DJ, Sabata M, Alfonso F, Macaya C (2004) “Candy wrapper” effect after drug-eluting stent implantation: déjà vu or stumbling over the same stone again? Catheter Cardiovasc Interv 61(3):387–391. https://doi.org/10.1002/ccd.10765
Kang SJ, Mintz GS, Akasaka T et al (2011) Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation 123(25):2954–2963. https://doi.org/10.1161/CIRCULATIONAHA.110.988436
Divakaran S, Loscalzo J (2017) The role of nitroglycerin and other nitrogen oxides in cardiovascular therapeutics. J Am Coll Cardiol 70(19):2393–2410. https://doi.org/10.1016/j.jacc.2017.09.1064
Bauer JA, Fung HL (1991) Differential hemodynamic effects and tolerance properties of nitroglycerin and an S-nitrosothiol in experimental heart failure. J Pharmacol Exp Ther 256(1):249–254. https://doi.org/10.0000/PMID1899118
Garg UC, Hassid A (1989) Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83(5):1774–1777. https://doi.org/10.1172/JCI114081
Kowaluk EA, Fung HL (1990) Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther 255(3):1256–1264
Kowaluk EA, Poliszczuk R, Fung HL (1987) Tolerance to relaxation in rat aorta: comparison of an S-nitrosothiol with nitroglycerin. Eur J Pharmacol 144(3):379–383. https://doi.org/10.1016/0014-2999(87)90392-x
Shaffer JE, Han BJ, Chern WH, Lee FW (1992) Lack of tolerance to a 24-hour infusion of S-nitroso N-acetylpenicillamine (SNAP) in conscious rabbits. J Pharmacol Exp Ther 260(1):286–293. https://doi.org/10.1002/jps.2600810124
Lindkvist M, Fernberg U, Ljungberg LU et al (2019) Individual variations in platelet reactivity towards ADP, epinephrine, collagen and nitric oxide, and the association to arterial function in young, healthy adults. Thromb Res 174:5–12. https://doi.org/10.1016/j.thromres.2018.12.008
Shen YH, Wang XL, Wilcken DE (1998) Nitric oxide induces and inhibits apoptosis through different pathways. FEBS Lett 433(1–2):125–131. https://doi.org/10.1016/S0014-5793(98)00844-8
Ahmed FE, Lalia BS, Hashaikeh R (2015) A review on electrospinning for membrane fabrication: challenges and applications. Desalination 356:15–30. https://doi.org/10.1016/j.desal.2014.09.033
Mo XM, Xu CY, Kotaki M, Ramakrishna S (2004) Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials 25(10):1883–1890. https://doi.org/10.1016/j.biomaterials.2003.08.042
Burger C, Hsiao BS, Chu B (2006) Nanofibrous materials and their applications. Annu Rev Mater Res 36:333–368. https://doi.org/10.1146/annurev.matsci.36.011205.123537
Zhang Y, Fang Q, Niu K et al (2019) Time-dependently slow-released multiple-drug eluting external sheath for efficient long-term inhibition of saphenous vein graft failure. J Contr Release 293:172–182. https://doi.org/10.1016/j.jconrel.2018.12.001
Ju YM, Choi JS, Atala A et al (2010) Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 31(15):4313–4321. https://doi.org/10.1016/j.biomaterials.2010.02.002
Zhang H, Jia X, Han F et al (2013) Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 34(9):2202–2212. https://doi.org/10.1016/j.biomaterials.2012.12.005
Al-Sa’doni H, Ferro A (2000) S-Nitrosothiols: a class of nitric oxide-donor drugs. Clin Sci 98(5):507–520. https://doi.org/10.1042/cs0980507
Naghavi N, de Mel A, Alavijeh OS et al (2013) Nitric oxide donors for cardiovascular implant applications. Small 9(1):22–35. https://doi.org/10.1002/smll.201200458
Joner M, Nakazawa G, Finn AV et al (2008) Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol 52(5):333–342. https://doi.org/10.1016/j.jacc.2008.04.030
Lipke EA, West JL (2005) Localized delivery of nitric oxide from hydrogels inhibits neointima formation in a rat carotid balloon injury model. Acta Biomater 1(6):597–606. https://doi.org/10.1016/j.actbio.2005.07.010
Funai EF, Davidson A, Seligman SP, Finlay TH (1997) S-nitrosohemoglobin in the fetal circulation may represent a cycle for blood pressure regulation. Biochem Biophys Res Commun 239(3):875–877. https://doi.org/10.1006/bbrc.1997.7565
Minamiyama Y, Takemura S, Inoue M (1997) Effect of thiol status on nitric oxide metabolism in the circulation. Arch Biochem Biophys 341(1):186–192. https://doi.org/10.1006/abbi.1997.9956
Bauer JA, Fung HL (1991) Chemical stabilization of a vasoactive S-nitrosothiol with cyclodextrins without loss of pharmacologic activity. Pharm Res 8:1329–1334. https://doi.org/10.1023/A:1015824417569
Kowaluk EA, Fung HL (1990) Dissociation of nitrovasodilator-induced relaxation from cyclic GMP levels during in vitro nitrate tolerance. Eur J Pharmacol 176:91–95. https://doi.org/10.1016/0014-2999(90)90136-T
Münzel T, Daiber A, Mülsch A (2005) Explaining the phenomenon of nitrate tolerance. Circ Res 97(7):618–628. https://doi.org/10.1161/01.RES.0000184694.03262.6d
Lautner G, Meyerhoff ME, Schwendeman SP (2016) Biodegradable poly(lactic-co-glycolic acid) microspheres loaded with S-nitroso-N-acetyl-D-penicillamine for controlled nitric oxide delivery. J Contr Release 225:133–139. https://doi.org/10.1016/j.jconrel.2015.12.056
Farah C, Michel LYM, Balligand JL (2018) Nitric oxide signalling in cardiovascular health and disease. Nat Rev Cardiol 15(5):292–316. https://doi.org/10.1038/nrcardio.2017.224
Kinlay S, Behrendt D, Wainstein M et al (2001) Role of endothelin-1 in the active constriction of human atherosclerotic coronary arteries. Circulation 104(10):1114–1118. https://doi.org/10.1161/hc3501.095707
Davignon J (2004) Role of endothelial dysfunction in atherosclerosis. Circulation 109(23_suppl_1):III-27-III–32
Drexler H (1998) Factors involved in the maintenance of endothelial function. Am J Cardiol 82(10A):3S-4S. https://doi.org/10.1016/S0002-9149(98)90420-9
Gimbrone MA, García-Cardeña G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118(4):620–636. https://doi.org/10.1161/CIRCRESAHA.115.306301
Hansson GK (2001) Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 21(12):1876–1890. https://doi.org/10.1161/hq1201.100220
Steinberg D (2009) The LDL modification hypothesis of atherogenesis: an update. J Lipid Res 50(Suppl):S376–S381. https://doi.org/10.1194/jlr.R800087-JLR200
Acknowledgements
This work was supported by the Natural Key Research and Development Project of Zhejiang Province, China (No. 2018C03015), the National Key Research and Development Program of China (No. 2016YFC1102203), and the Medical Health Science and Technology Projects of Zhejiang Province (No. 2019KY426).
Author information
Authors and Affiliations
Contributions
SYC and FJ contributed to conceptualization; SYC and LYZ were involved in methodology; FJ contributed to investigation; SYC was involved in writing-original draft; all authors contributed to writing-review and editing; FYQ, JJ, GSF were involved in funding acquisition; SHJ, JJ, GSF contributed in resources; SHJ, JJ, GSF were involved in supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, S., Jia, F., Zhao, L. et al. Electrospun fiber membrane with asymmetric NO release for the differential regulation of cell growth. Bio-des. Manuf. 4, 469–478 (2021). https://doi.org/10.1007/s42242-021-00131-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-021-00131-w