Abstract
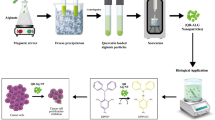
The present study involves the preparation, characterization, and evaluation of novel alginate nanocarrier for sunitinib (STB), an anticancer drug. Sunitinib (STB) is a weakly soluble drug in water due to its poor dissolution rate and oral bioavailability. Alginate biopolymer is one of the promising candidates for a delivery matrix. A controlled gelification method using calcium chloride as the crosslinker was done for the formulation of STB using nanoalginate. The objective of this work was to evaluate the physicochemical/biological properties of the release system via in vitro dissolution studies and cytotoxicity studies. The bare alginate nanoparticles and STB-loaded alginate nanoparticles were characterized for its physicochemical properties using FT-IR, SEM, and TEM studies. The formulation and loading chemistry were well exhibited by FTIR analysis results. SEM and TEM results revealed the spherical and rod-like shape morphology with rough surface where the drug molecules were adhered. The entrapment efficiency and dissolution studies of the drug were done through UV VIS instrument. In vitro cytotoxic studies were performed for loaded sunitinib alginate biopolymer (STB-AL-NPs). The cytotoxicity results reveal that the cytotoxicity is mainly due to the loaded anticancer drug sunitinib.
Lay Summary
In this study, sunitinib drug was encapsulated in alginate nanoparticles effectively. The interaction between polymer, crosslinker, and drug was well determined using FTIR, XRD, SEM, TEM, and Zeta potential. The results are compared with bare alginate nanoparticles. The expected entrapment efficiency of the drug carrier was around 99% via UV-Vis spectroscopy, proving good drug-polymer interaction. In vitro drug release studied was done by dissolution method using 900 mL of 0.2 mM PBS, to determine the sustained release of the drug from the excipient. The results proved that the drug was released to the medium in the sustained manner.
Future Scope
Sunitinib-encapsulated alginate nanoparticles not only offer several advantages over conventional drug therapies but also expected to overcome the side effect regarding to dosing and toxicity while administration. However, further optimization studies includes stabilization and targeting should be performed for both in vitro and in vivo.








Similar content being viewed by others
References
Mokhtarzadeh A, Alibakhshi A, Hejazi M, Omidi Y, Ezzati Nazhad Dolatabadi J. Bacterial-derived biopolymers: advanced natural nanomaterials for drug delivery and tissue engineering. Trends Anal Chem. 2016a;82:367–84.
Deepu S, Mathew M, Shamna MS. Controlled drug delivery system: review. Int J Pharm Chem Sci. 2014;3(3):636–41.
Mokhtarzadeh A, Alibakhshi A, Yaghoobi H, Hashemi M, Hejazi M, Ramezani M. Recent advances on biocompatible and biodegradable nanoparticles as gene carriers. Expert Opin Biol Ther. 2016b;16:771–85.
Peralta-Yahya PP, Zhang F, Del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nature. 2012;488:320–8. https://doi.org/10.1038/nature11478.
Jana S, Gandhi A, Sen KK. BasuSk, natural polymers and their application in drug delivery and biomedical field. J Pharm Sci Technol. 2011;1(1):16–27 http://www.pharmascitech.in/admin/php/uploads/6_pdf.pdf.
Liu W, Li Y, Liu J, Niu X, Wang Y, Li D. Review Article Application and performance of 3D printing in nanobiomaterials. J Nanomater. 2013;7 pages. https://doi.org/10.1155/2013/681050.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–26. https://doi.org/10.1016/j.progpolymsci.2011.06.003.
Taha MO, Nasser W, Ardakani A, AlKhatib HS. Sodium lauryl sulfate impedes drug release from zinc-crosslinked alginate beads: switching from enteric coating release into biphasic profiles. Int J Pharm. 2008;350:291–300.
Pereira R, Mendes A, Bártolo P. Alginate/aloe vera hydrogel films for biomedical applications. Procedia CIRP. 2013;5:210–5. https://doi.org/10.1016/j.procir.2013.01.042.
Fischer FG, Dörfel H. Die polyuronsauren der braunalgen-(kohlenhydrate der algen-I). Z Physiol Chem. 1955;302:186–203. https://doi.org/10.1007/978-94-009-1836-8.
Mørch YA, Donati I, Strand BL, Skjåk-Braek G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7(5):1471–80. https://doi.org/10.1021/bm060010d.
Rousseau I, Le Cerf D, Picton L, Argillier JF, Muller G. Entrapment and release of sodium polystyrene sulfonate (SPS) from calcium alginate gel beads. Eur Polym J. 2004;40:2709–15.
Fang Y, Al-Assaf S, Phillips GO, Nishinari K, Funami T, Williams PA, et al. Multiple steps and critical behaviors of the binding of calcium to alginate. J Phys Chem B. 2007;111:2456–62.
Kondaveeti S, Cornejo DR, Petri DFS. Alginate/magnetite hybrid beads for magnetically stimulated release of dopamine. Colloids Surf B: Biointerfaces. 2016;138:94–101.
Arora S, Gupta S, Narang RK, Budhiraja RD. Amoxicillin loaded chitosan-alginate polyelectrolyte complex nanoparticles as mucopenetrating delivery system for H. pylori. Sci Pharm. 2011;79(3):673–94.
Ahmad Z, Pandey R, Sharma S, Khuller GK. Pharmacokinetic and pharmacodynamic behaviour of antitubercular drugs encapsulated in alginate nanoparticles at two doses. Int J Antimicrob Agents. 2006;27(5):420–7.
Borges O, Silva M, de Sousa A, Borchard G, Junginger HE, Cordeiro-da-Silva A. Alginate coated chitosan nanoparticles are an effective subcutaneous adjuvant for hepatitis B surface antigen. Int Immunopharmacol. 2008;8(13–14):1773–80.
Jie Hu MS, Yujin Zong MD, Jun Li BS, Xiaodong Zhou MD, Jun Zhang MD, Ting Zhu MD, et al. In vitro and in vivo evaluation of targeted sunitinib-loaded polymer microbubbles against proliferation of renal cell carcinoma. J Ultrasound Med. 2016;35:589–97.
Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate. J Pharm Sci. 1993;82(9):912–7. https://doi.org/10.1002/jps.2600820909.
Albertoni F, Pettersson B, Reichelova V, Juliusson G, Liliemark J. Analysis of2-chloro-2′-deoxyadenosine in human blood plasma and urine byhigh-performance liquid chromatography using solid-phase extraction. Ther Drug Monit. 1994;16:413–8.
Gomathi T, Sudha PN, Florence JAK, Venkatesan J, Anil S. Fabrication of letrozole formulation using chitosan nanoparticlesthrough ionic gelation method. Int J Biol Macromol. 2017;104 (1820–32.
Hadjiioannou TP, Christian GD, Koupparis MA, Macheras PE. Quantitative calculations in pharmaceutical practice and research. New York: VCH Publishers Inc.; 1993. p. 345–8.
Bourne DWA. Pharmacokinetics. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. 4th ed. New York: Marcel Dekker Inc.; 2002. p. 67–92. http://www.ajprd.com/downloadebooks_pdf/32.pdf.
Higuchi TJ. Mechanism of sustained medication. Theoretical analysis of rateof release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;84:1464–77. https://doi.org/10.1002/jps.2600521210.
Hixson AW, Crowell JH. Dependence of reaction velocity upon surface andagitation (I) theoretical consideration. Ind Eng Chem. 1931;23:923–31. https://doi.org/10.1021/ie50260a018.
Sarmento B, Ferreira D, Veiga F, Ribeiro A. Characterization of insulin-loaded alginate nanoparticles producedbyionotropic pre-gelation through DSC and FTIR studies. Carbohydr Polym. 2006;66:1–7. https://doi.org/10.1016/j.carbpol.2006.02.008.
Gomathi T, Govindarajan C, Maximas RHR, Sudha PN, Mohamed Imran PK, Venkatesan J, et al. Studies on drug-polymer interaction, in vitro release and cytotoxicity from chitosanparticles excipient. Int J Pharm. 2014;468:214–22. https://doi.org/10.1016/j.ijpharm.2014.04.026.
Joseph JJ, Sangeetha D, Gomathi T. Sunitinib loaded chitosan nanoparticles formulation and its evaluation. Int J Biol Macromol. 2016;82:952–8. https://doi.org/10.1016/j.ijbiomac.2015.10.079.
Yang SJ, Lin FH, Tsai HM. Alginate-folic acid-modified chitosan nanoparticles for photodynamic detection of intestinal neoplasms. Biomaterials. 2011;32(8):2174–82. https://doi.org/10.1016/j.biomaterials.2010.11.039.
Yotsuyanagi T, Ohkubo T, Ohhashi T, Ikeda K. Calcium-induced gelatin of alginic acid and pH-sensitive reswelling of dried gels. Chem Pharm Bull. 1987;35:1555–63.
Aydm STR, Pulat M. 5-fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: evaluation of controlled release kinetics. J Nanomater. 2012:1–10.
Sriamornsak P, Thirawong N, Korkerd K. Swelling, erosion and release behaviour of alginate-based matrix tablets. Eur J Pharm Biopharm. 2007;66:435–50. https://doi.org/10.1016/j.ejpb.2006.12.003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joseph, J.J., Sangeetha, D. & Shivashankar, M. In vitro Release and Cytotoxic Studies of Novel Alginate Nanocarrier for the Antitumor Drug: Sunitinib. Regen. Eng. Transl. Med. 5, 220–227 (2019). https://doi.org/10.1007/s40883-018-0090-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-018-0090-y