Abstract
The graphitic carbon nitride (g-C3N4) which is a two-dimensional conjugated polymer has drawn broad interdisciplinary attention as a low-cost, metal-free, and visible-light-responsive photocatalyst in the area of environmental remediation. The g-C3N4-based materials have excellent electronic band structures, electron-rich properties, basic surface functionalities, high physicochemical stabilities and are “earth-abundant.” This review summarizes the latest progress related to the design and construction of g-C3N4-based materials and their applications including catalysis, sensing, imaging, and white-light-emitting diodes. An outlook on possible further developments in g-C3N4-based research for emerging properties and applications is also included.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Highlights
-
The g-C3N4-based materials have excellent electronic band structures, electron-rich properties, basic surface functionalities, high physicochemical stabilities and are “earth-abundant.”
-
Recent progress of g-C3N4-based nanostructures in catalyst, sensing, imaging and LEDs have been discussed in details.
-
An outlook on possible further developments in g-C3N4-based research for emerging properties and applications is also included.
2 Introduction
Graphitic carbon nitride (g-C3N4), one of the oldest reported polymers in the literature, has a general formula of (C3N3H)n. The history of development could be traced back to 1834 [1]. Research work has been inspired in the 1990s due to a theoretical prediction that diamond-like β-C3N4 could have extremely high hardness values [2]. At ambient conditions, g-C3N4 is regarded as the most stable allotrope. Similar to graphite, g-C3N4 is a layered material in which van der Waals force holds the stacking layers (covalent C–N bonds) and each layer is composed of tri-s-triazine units connected with planar amino groups [3]. The tri-s-triazine ring structure provides the polymer a high thermal stability (600 °C in air) and chemical stability in both acidic and alkaline environments [4].
Utilization of g-C3N4 in the heterogeneous catalysis arena started around a decade ago [5, 6]. The discovery of g-C3N4 polymer as a metal-free conjugated semiconductor photocatalysis for water splitting was first reported by Wang et al. [7] due to its appealing electronic structure, i.e., having a modulated bandgap and being an indirect semiconductor. Since then, these unique properties of g-C3N4 make it a promising candidate for visible-light photocatalytic applications utilizing solar energy. Solar energy is attracting worldwide attention by providing about 120,000 TW annually to the earth as one of the green, clean, and sustainable energy resources. Solar-induced chemical processes would be able to greatly extend the applications of g-C3N4. Since the landmark discovery of photocatalytic water splitting using TiO2 electrodes by Fujishima in 1972, photocatalytic technology has been regarded as one of the most important strategies to address global energy and environmental issues [8]. Since then, there have been numerous developments in the fabrication of highly efficient semiconductor-based photocatalysts such as metal-based oxides and sulfides [9,10,11,12].
Notably, g-C3N4 has become a new family of next generation, non-toxic, metal-free, earth-abundant, and visible-light-driven polymeric semiconductor for applications in the degradation of organic pollutants, hydrogen evolution from water, sensing, imaging, and energy conversion [5, 9, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144]. Many reviews can be found mainly focusing on synthesis and catalytic applications of g-C3N4 [39,40,41,42,43,44,45, 145, 146]. However, a systematic description of the catalyst (photo and organic), bio-imaging, (chemical and bio-) sensing, devices, and energy-related applications (batteries, supercapacitors, white-light-emitting diodes, and oxygen reduction reaction) of g-C3N4 has not been presented until now. In this review, we give an overview of the porosity, luminescence, conductivity, and catalytic properties of g-C3N4, as well as their bio-imaging, photodynamic therapy, chemical sensing, and white-light-emitting diode applications. We believe this is the dawn for the development of g-C3N4. There are still new physical properties yet to be discovered based on g-C3N4 nanostructures. We are at a critical time to highlight the progress and provide a good source of references for this booming research topic.
3 g-C3N4-Based Structures
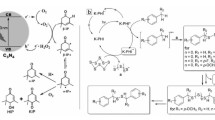
First-principle calculations predicted seven phases of g-C3N4, namely α-C3N4 (bandgap of 5.5 eV), β-C3N4 (bandgap of 4.85 eV), cubic C3N4 (bandgap of 4.3 eV), pseudocubic C3N4 (bandgap of 4.13 eV), g-h-triazine (bandgap of 2.97 eV), g-o-triazine (bandgap of 0.93 eV), and g-h-heptazine (bandgap of 2.88 eV) [4]. Figure 1 shows the primary tectonic units, triazine and tri-s-triazine ring structures, for forming the allotropes of g-C3N4. The structure can be viewed as graphite whose carbon lattice is partially substituted with nitrogen atoms in a regular fashion. g-C3N4 is an n-type semiconductor [5,6,7], which intrinsically possesses a very high nitrogen content dominated by a pyridinic and graphitic nitrogen while supported by a two-dimensional (2D) graphene sheet or three-dimensional (3D) porous graphitic carbon. The structure of g-C3N4 can be controlled by a variety of synthetic routes, including different condensation temperature, different ratio of precursors, porosity induced by hard/soft templating strategies, and exfoliation and doping. Synthetic routes and various morphologies including bulk, mesoporous, 3D, 2D, one-dimensional (1D), and zero-dimensional (0D) g-C3N4 will be discussed in the following.
a Triazine and b tri-s-triazine (heptazine) structures of g-C3N4. Reprinted with permission from Ref. [40]. Copyright 2016 American Chemical Society
3.1 Synthetic Routes of g-C3N4
g-C3N4 can be synthesized by thermal polymerization of abundant nitrogen-rich and oxygen-free compound precursors containing pre-bonded C–N core structures (triazine and heptazine derivatives) such as urea [46], melamine [47,48,49], dicyandiamide [50,51,52,53,54], cyanamide [14, 35, 55, 56], thiourea [57, 58], guanidinium chloride [59,60,61, 125], guanidine thiocyanate [126], and thiourea oxide [127]. The condensation pathways from above C/N precursors are facile and efficient routes to form the polymeric g-C3N4 network [39]. Many reports discussed that different types of precursors and treatments can strongly influence the physicochemical properties of the resulting g-C3N4, including surface area, porosity, absorption, photoluminescence, C/N ratio, and nanostructures.
Various surface modifications and functionalities have been employed to obtain desired structures such as 3D bulks, 2D nanosheets, 2D films, 1D nanorods, 1D nanotubes, 1D nanowires, and 0D quantum dots. For example, the urea precursor can be transformed to g-C3N4 at ca. 550 °C, as confirmed by X-ray diffraction (XRD, Fig. 2a, b). The C3N4 powders are usually yellow under the visible light. The optical properties are shown in Fig. 2c. The polymeric g-C3N4 is unstable at above 600 °C. Beyond 700 °C, g-C3N4 produces nitrogen and cyano fragments.
Crystal structure and optical properties of graphitic carbon nitride. a Schematic diagram of a perfect graphitic carbon nitride sheet constructed from melem units. b Experimental XRD pattern of the polymeric carbon nitride, revealing a graphitic structure with an interplanar stacking distance of aromatic units of 0.326 nm. c UV-visible diffuse reflectance spectrum and image (inset) of g-C3N4. Reprinted with permission from Ref. [5]. Copyright 2009 Nature Publishing Group. Representation of the β-C3N4 (d), α-C3N4 (e), graphite-C3N4 (f), pseudocubic-C3N4 (g), and cubic-C3N4 (h). The carbon and nitrogen atoms are depicted as gray and blue spheres, respectively, from Ref. [4]. Copyright 1996 American Association for the Advancement of Science. (Color figure online)
General analytical techniques for confirming the presence of g-C3N4 include X-ray photoelectron spectroscopy (XPS), XRD, and Fourier transform infrared (FTIR) spectroscopy [40]. The density function theory (DFT) calculations are used to reveal the characteristics of the valence and conduction bands. For example, g-C3N4 is found to mainly composed of the nitrogen p Z orbitals and carbon p Z orbitals, respectively [5].
3.2 Porosity of g-C3N4
High surface area and continuous porosity (as active centers) are important requirements for catalysis, gas, and energy storage technologies. Introduction of porosity in g-C3N4 can significantly increase their exposed surface area and accessible channels, and active edges, thus promoting the charge separation, molecular mass transfer, light harvesting, and surface reactions [145]. All these advantageous features can benefit the enhancement of photocatalytic efficiency. It is well known that the porous carbons have outstanding properties with respect to their use in energy applications, including as electrode materials for supercapacitors and as materials for solid-state hydrogen and carbon dioxide storage. The attractive attributions of porous carbons include low cost, environmental friendliness, chemical and thermal stability, easiness of processing, and low framework density. The activated carbons have been traditionally employed as absorbents or catalyst supports [62]. Compared with activated carbons, g-C3N4 has a moderate nitrogen content and ideal stoichiometry. The nitrogen content induces their unique surface properties such as semiconducting character, mechanical stability, thermal and chemical stability, which are superior to all other carbon nanomaterials (Table 1).
Cavities can be introduced to form mesoporous g-C3N4 frameworks [29]. Qiao’s group reported the preparation of flexible films by integrating 2D mesoporous g-C3N4 (SiO2 template method) with graphene sheets. A commonly used mesoporous silica, SBA15 was employed as a template to fabricate mesoporous g-C3N4 [6, 56]. However, hydrogen fluoride or other acid that are usually used to remove the host matrices of SiO2 is hazardous. Besides hard template methods [128,129,130], soft templates [131, 132] and bubble templates [133,134,135,136] are also utilized to fabricate porous g-C3N4. Liu et al. reported the preparation of porous g-C3N4 by a simple co-pyrolyzation of co-precursors melamine and NH4HCO3. NH4HCO3 not only enhanced the specific area by bubbles, but it also shifted the conduction band and promoted the separation of charge carriers [63]. Calcium salts have also been utilized as a template for the synthesis of porous g-C3N4 with enhanced surface properties [143]. Metal–organic framework (MOF) is a typical high surface area materials and has also been utilized as templates for porous g-C3N4. Pandiaraj et al. [64] have reported MOF-derived g-C3N4 porous nanostructures.
3.3 Bulky g-C3N4
Bulky g-C3N4 can be synthesized by thermal condensation of a variety of precursors such as cyanamide, dicyandiamide, melamine, thiourea or urea between 400 and 600 °C. For example, g-C3N4 was synthesized from cyanamide into a combination of addition and polycondensation, in which case the cyanamide molecules were condensed to dicyandiamide and melamine at 203 and 234 °C, respectively. Next, the condensed dicyandiamide was removed. Essentially, all melamine-based products were found when the temperature was about 335 °C. Further heating to about 390 °C resulted in the rearrangement of tri-mesotriazine units via melamine. Finally, the polymer g-C3N4 occurred at about 520 °C by further condensation of the unit, which is thermally unstable at temperatures above 600 °C. During the calcination, the color changed from white (precursor) to light yellow (500 °C) and then dark orange (650 °C) [5, 40]. However, g-C3N4 obtained by such methods usually possesses a relatively low surface area (10 m2 g−1) and poor water solubility. Furthermore, the bulk g-C3N4 does not exhibit any photoluminescence characteristics when dissolved in solvents.
It has recently been found that urea is an excellent precursor for the synthesis of flaky g-C3N4 having a high specific surface area and a high porosity. g-C3N4 was synthesized at various calcination temperatures between 450 and 600 °C in a muffle furnace for 2 h at a heating rate of 15 °C min−1 from oxygen-containing urea. During the thermal treatment process, the generated gases such as NH3 at a low temperature (<200 °C) and CO2 at a high temperature play a leading role in processing highly porous g-C3N4. The advantage of this method includes simplicity, convenience, and the absence of introduction of impurities during the synthesis of nanostructures. Compared to urea-derived g-C3N4, comprising of wrinkled porous 2D nanosheets, both thiourea-derived and dicyandiamide-derived g-C3N4 samples showed large sheets without porous structure. The specific surface area and crystallinity of g-C3N4 were marginally improved with increasing calcination temperatures. Generally, the heating rate is slower; the porosity of g-C3N4 could be improved [40].
Exfoliation methods such as sonication have been employed as a typical top-down route to obtain ultrathin g-C3N4 with excellent photoluminescence properties [65]. Bulk materials can be swelled and then exfoliated in the pure water. The dispersed ultrathin g-C3N4 nanosheets were negatively charged (zetapotential of about ~30.3 mV).
3.4 Three-Dimensional g-C3N4-Based Micro/Nanostructures
Three-dimensional architectures fabricated using nano-scale building blocks (0D, 1D, and 2D) are hot topics due to the desirable combination of high internal reactive surface area and straightforward molecular transport. However, the fabrication of 3D porous g-C3N4 has been a big challenge up to now. Recently, Liu’s group developed an efficient chemical vapor deposition growth strategy for 3D g-C3N4/graphene nanocomposites [66]. They found that g-C3N4 can be grown along the surface of graphene (see Fig. 3a, b, c). The C–C bonds (284.7 eV) and N–C=N bonds (288.3 eV) can be detected from the high-resolution C 1 s spectrum (Fig. 3d). Furthermore, sp2 aromatic C=N–C (398.8 eV), tertiary N-(C)3 (400.1 eV), and amino group C–N–H (401.3 eV) can be fitted in the high-resolution N 1 s spectrum (Fig. 3e). UV–vis spectroscopy (Fig. 3f) reveals the optical properties of the 3D composites to be similar to those of the pure g-C3N4.
a Schematic 3D g-C3N4/Graphene structures. b, c SEM images of g-C3N4/graphene nanocomposites. Inset TEM image. d C 1s, e N 1s XPS and f UV–vis spectra of g-C3N4/graphene nanocomposites. Insets: photographs of the powder samples. Reprinted with permission from Ref. [66]. Copyright 2016 American Chemical Society
Yuan et al. [67] reported the 3D porous g-C3N4 network assembled by exfoliated ultrathin nanosheets interconnected in large quantity via H2SO4 intercalation and subsequent thermal treatment.
3.5 Two-Dimensional g-C3N4 Nanostructures
Two-dimensional materials have received tremendous attention in the past decade because their ultimate structure was reported by Geim [69]. The topic about graphene has been cited over 30,000 times up to now (Google Scholar), and graphene has been employed widely in energy applications (such as lithium-ion batteries and supercapacitors [70,71,72]). Similar to graphene, g-C3N4 also has a typical sp2 network (graphite-like layer structure) with weak van der Waals interactions across the layers. Inspired by the successful exfoliation of graphene from bulk graphite, Xie et al. [65] firstly demonstrate that ultrathin g-C3N4 nanosheets could be prepared by a green liquid exfoliation from bulk g-C3N4 in water. From bulk to ultrathin nanosheets (several layers), g-C3N4 nanosheets show an obvious increase in density of states (DOS) at the conduction band edge with respect to the bulk counterpart by first-principle density-functional calculations. Global effects have been launched to synthesize single or few-layer g-C3N4 nanosheets due to their attractive physicochemical properties [52, 73, 74]. Figure 4 shows characterization of the g-C3N4 nanosheets studied by transmission electron microscopy (TEM) and atomic force microscopy (AFM), illustrating the similarity of ultrathin layers of exfoliated g-C3N4 to that of graphene.
a-c TEM images and d AFM image of g-C3N4 nanosheets. Inset of c SAED pattern. Reprinted with permission from Ref. [52]. Copyright 2014 John Wiley and Sons. e Schematic illustration of top-down and bottom-up synthetic strategies for g-C3N4 nanosheets. Reprinted with permission from Ref. [68]. Copyright 2015 The Royal Society of Chemistry. (Color figure online)
There are two other synthetic approaches of 2D g-C3N4 nanosheets: one is known as the top-down approach, and the other one is the bottom-up approach (Fig. 4e). For the top-down approach, chemical etching and ultrasonication-assisted liquid exfoliation are the two main technologies involved [75, 76]. Template-assisted method and heteroatom-mediated method have been commonly employed for the bottom-up approaches [77, 78]. The big atomic size of sulfur can influence the conformation and the connectivity of the resultant g-C3N4 and hence offer a template tool to tune the texture and electronic structure. The first report of template-mediate synthesis of 2D g-C3N4 nanosheets involved the use of GO-derived silica [78]. Zhang et al. [23] reported the sol processing for the fabrication of g-C3N4 thin films with HNO3 as a strong oxidizing acid. Liu et al. [54] developed a method to grow g-C3N4 thin films directly on conductive substrates.
3.6 One-Dimensional g-C3N4 Nanostructure (Nanorods, Nanotube, and Nanofibers)
One-dimensional g-C3N4 nanostructures hold good promise for electronic and electrochemical performances due to their high surface area, and light harvesting and mass transfer properties. Wang et al. described a facile method to fabricate g-C3N4 nanotubes (Fig. 5a) by directly heating melamine without any templates. The resulting nanotubes exhibited a blue fluorescence and excellent photocatalytic properties [79]. Hollow 1D g-C3N4 nanostructures have been developed using a sulfur-mediated self-templating method by Liu’s group [83], as shown in Fig. 5b.
1D g-C3N4 nanostructures. a SEM image of nanotubes [79]. b TEM image of nanotubes [83]. c TEM image of nanorods [81]. d TEM image of porous nanorods [82]. Inset: pore size distribution. e SEM image of nanofibers [30]. f SEM image of tubular structures [85]. Reprinted with permission from Ref. [79] (Copyright 2014 The Royal Society of Chemistry), with permission from Ref. [83] (Copyright 2016 American Chemical Society), with permission from Ref. [81] (Copyright 2014 American Chemical Society), with permission from Ref. [82] (Copyright 2012 The Royal Society of Chemistry), with permission from Ref. [30] (Copyright 2013 American Chemical Society), with permission from Ref. [85] (Copyright 2016 Wiley–VCH Verlag GmbH), respectively
Liu et al. [81] also reported the synthesis of g-C3N4 nanorods by using chiral silica nanorods as templates for practical enzymatic applications. In addition, Li et al. [82] described a one-step method to fabricate mesoporous g-C3N4 nanorods with the template SBA-15. Tahir et al. [30] reported the synthesis of g-C3N4 nanofibers for energy storage and Photo degradation applications. Recently, Tong et al. combined the hydrothermal and condensation techniques to obtain a tubular g-C3N4 isotype heterojunction with excellent photocatalytic property [85]. Figure 5c–f depicts these tubular nanostructures.
Zheng et al. [80] developed a nanocasting technique to fabricate twisted hexagonal rod-like C3N4 by using chiral silicon dioxide as templates. The helix is an important template in nature, as one finds it in DNA, RNA, and proteins. Synthesis of chiral inorganic nanostructures has recently gained considerable attention. The chiral nanostructures shown in Fig. 6a–f reveal the helical morphology and ordered channels winding around the rod centers. This is the first report about both left- and right-handed chiral nanostructures with unique optical activities.
Morphology characterization of the HR-CN sample. a SEM, b TEM, and c, d corresponding elemental mapping images of g-C3N4. Reproduced from Ref. [80] by permission of the John Wiley & Sons Ltd. e A g-C3N4 layer and f A single g-C3N4 nanotube formed by rolling the g-C3N4 layer. Reproduced from Ref. [79] by permission of the Royal Society of Chemistry
3.7 Zero-Dimensional g-C3N4 Nanostructure
When the size of the nanostructure is less than 10 nm, g-C3N4 nanostructures (typically contain a few thousand atoms) usually show significant quantum confinement effects and possess excellent properties like bright fluorescence, water solubility, and above all, non-toxicity. This is in contrast to the fact that most essential elements in semiconductor quantum dots (QDs) (for example, Se in CdSe, Pb in PbTe, and Te in CdTe) all have risks of long-term toxicity and potential environmental hazards. Similar to the synthetic routes of 2D g-C3N4 nanosheets, g-C3N4 QDs have been mainly synthesized by top-down and bottom-up approaches.
g-C3N4 QDs were prepared first by the top-down approach. Wang et al. was the first to prepare g-C3N4 QDs by a thermal-chemical etching process from bulk g-C3N4, as illustrated in Fig. 7e [86]. Xie et al. demonstrated an exfoliation strategy for the preparation of single-layered QDs. When these QDs passed through the cell membranes, they exhibited an excellent two-photon absorption behavior as compared with the double-layered QDs [87] (Fig. 7a–d). Recently, Wu et al. developed a doping method (phosphorus as dopant) to control the emission wavelength of g-C3N4 QDs to be in the whole visible-light regime [88]. This was the first report that phosphorus doping could change the direct bandgap of g-C3N4 QDs.
a HOMO-n and b LUMO + n orbitals of the single-layered g-C3N4, respectively. c HOMO-n and d LUMO + n orbitals of the double-layered g-C3N4, respectively. Reproduced from Ref. [87] by permission of John Wiley & Sons Ltd. e Schematic illustration of the controllable synthesis of g-C3N4 nanosheets, nanoribbons, and quantum dots. Reproduced from Ref. [86] by permission of the Royal Society of Chemistry
Hydrothermal and microwave heating methods are commonly utilized for the synthesis of g-C3N4 QDs as the bottom-up approach [89, 90]. Lu et al. [90] reported the synthesis of g-C3N4 QDs with strong blue photoluminescence by hydrothermal heating of the citric acid and thiourea. Cao et al. [89] developed a facile microwave-assisted fabrication of g-C3N4 QDs. Compared with the majority of carbon materials such as graphene QDs and carbon QDs, g-C3N4 QDs which possess both nitrogen-rich and electron-rich properties and basic surface functionalities represent a new family of luminescent QDs.
4 Multifunctional Applications
As the most stable allotrope of carbon nitride, g-C3N4 is versatile materials with unique semiconducting, excellent photocatalytic properties. They are also environmental friendly, low cost, and metal-free, which make them attractive for a range of applications beyond catalysis, including sensing, bio-imaging, photodynamic therapy, and energy conversion.
4.1 g-C3N4 Catalysts
Polymeric g-C3N4 semiconductors are widely used as catalysts due to their excellent chemical stability and unique electronic band structure. In the formation of the g-C3N4 network, the C-p z orbit composes the lowest unoccupied molecular orbital (LUMO), and N-p z orbital composes the highest occupied molecular orbital (HOMO), with a 2.7 eV bandgap between these two orbitals [91]. This suitable bandgap can absorb the solar electromagnetic energy with wavelength less than 475 nm. It was found that pyrrolic nitrogen has the strongest role in acetylene hydrochlorination among all nitrogen species [17].
The rapid recombination rate of electron–hole pairs results in low efficiency, thus limiting the practical applications of g-C3N4. Sun et al. [92] developed a homogeneous catalyst, Pd/g-C3N4, for a Suzuki–Miyaura coupling reaction with superior catalytic activity under mild conditions. It is well known that the Suzuki–Miyaura coupling reaction is of primary importance for the construction of C–C bonds. The uniform Pt nanoparticles deposited on the surface of g-C3N4 networks can result in a high yield of 97% biphenyl and 100% bromobenzene. Kumar et al. reported a nanocomposite of an iron(II) bipyridine with carbon nitride as a photocatalyst for the oxidative coupling of benzylamines under mild reaction conditions, resulting in excellent activity and effective recycling ability [93]. A photoactive catalyst Ru/g-C3N4 was developed by Sharma et al. [94] for efficient photocatalytic transfer hydrogenation of aldehydes and ketones under mild conditions. Xie’s group explored the generation of singlet oxygen in oxidized g-C3N4 [95].
g-C3N4 can be used as a new kind of metal-free photocatalysts. Wang et al. [5] was among the first to use g-C3N4 as a photocatalyst for hydrogen production from water. However, the quantum efficiency of the catalyst is only 0.1% with the irradiation of 420–460 nm due to its fast recombination of electron–hole pairs. To solve this problem, modified 2D g-C3N4 materials with a redshift absorption were produced and a quantum efficiency of 8.8% was achieved at 420 nm by the same group [96]. Liu et al. [97] developed a carbon dots/g-C3N4 nanocomposite as a metal-free photocatalyst with high quantum yield and excellent stability. The overall evolution of H2 and O2 is shown in Fig. 8a with a molar ratio of 2.02 (a value of 2 is identified for water splitting). Absorbance and quantum efficiency (QE) of the carbon dots/g-C3N4 nanocomposite were measured and are shown in Fig. 8b. The catalyst composition was optimized by measuring QE for different concentrations of carbon dots in a fixed mass of composite, as shown in Fig. 8c. With the increase in carbon dots, the QE can reach as high as 16% (Fig. 8d).
a Typical H2 and O2 production from water under visible-light irradiation. b Wavelength-dependent QE (red dots) of water splitting by composite catalyst. c QE for different concentrations of carbon dots/g-C3N4 catalysts in a fixed mass of composite catalyst. d QE for different catalyst loads with a constant carbon dot concentration in 150 ml of ultra-pure water. Reproduced with permission from Ref. [95]. Copyright 2015 American Association for the Advancement of Science. (Color figure online)
Recently, nanohybrids (van der Waals heterostructures) which compose of different 2D nanolayers exhibit much improved catalytic activities. For example, porous g-C3N4/graphene films have been fabricated as electrodes for efficient hydrogen evolution by Qiao et al. [29]. Dai et al. [98] obtained graphene oxide/g-C3N4 nanosheets by a sonication method with reinforced photocurrent. Ma et al. demonstrated the fabrication of hybrid g-C3N4/graphene quantum dot nanocomposites. The hybrid nanocomposites have an excellent efficiency for water splitting due to decreased bandgap. Experimental results and DFT calculations revealed that the chemical bonding of two different layered materials can improve the catalytic activity [18]. Xu et al. [137] fabricated AgBr/g-C3N4 hybrid materials with synergistic visible-light photocatalytic activity, and the uniform AgBr nanoparticles were well dispersed on the g-C3N4 nanosheets which enhanced the optical activity. What is more, many hybrid materials like g-C3N4/Ag2CO3 [138], g-C3N4/ZnWO4 [139], g-C3N4/Ag2O [140], and g-C3N4/Ag [141] were explored to enhance photocatalytic activity. Sun group obtained mesoporous carbon/g-C3N4 with remarkably enhanced photocatalytic activity due to enhanced visible light and dye adsorption [142]. Mesoporous g-C3N4 has been fabricated in the presence of SBA-15 with enhanced photocatalytic activity for methyl orange degradation by Gao et al. [144].
Crystalline carbon nitride nanosheets have been prepared by Lotsch et al. [76] to enhance visible-light hydrogen evolution. The authors stated that morphology and surface area are the two crucial factors governing the photocatalytic performances. An efficient deposition method of growing g-C3N4 on different electrodes has been developed by Shalom et al. [26]. The successful deposition technique enables the fabrication of many electronic devices based on g-C3N4. Wu et al. studied the effect of defects in g-C3N4 on hydrogen evolution and photovoltage. Controlling different types of defects is the key to improve the catalytic performance [27].
He et al. [20] utilized polydopamine/g-C3N4 composites to produce hydrogen from water with superior activity. DFT calculations were used to obtain the band structure of g-C3N4 with nonmetal element doping, including boron, oxygen, phosphorous, and others. Phosphorus was predicted to be suitable as a doping element in g-C3N4 because it can decrease the bandgap of g-C3N4 from 2.7 to 2.31 eV without any mid-gap states [28].
Shiraishi et al. [35] reported a g-C3N4 photocatalyst to reduce O2 to H2O2 via a two-electron route under visible light. Fang et al. [49] demonstrated that the nitrogen self-doped g-C3N4 can significantly enhance the catalytic activity (1.8 times) of hydrogen evolution and modify the optical and electronic properties with respect to the un-doped g-C3N4. Sun’s group reported ultrathin g-C3N4 nanosheets/graphene nanocomposites as a highly efficient electrocatalyst for oxygen evolution reaction. They revealed that the high oxygen evolution reaction activities resulted from pyridinic-N-related active sites [121]. They also developed 3D porous supramolecular architecture based on g-C3N4 nanosheets/graphene oxide as a highly efficient electrocatalyst for oxygen reduction reaction [122].
Besides metal-free materials such as graphene or carbon dots, various metal oxides and sulfides have been coupled with g-C3N4 for enhancing photocatalytic performances. For example, Chen et al. demonstrated that the g-C3N4/Ag/TiO2 heterostructure microspheres were successfully achieved with enhanced photocatalysis performances [36]. Gu et al. [100] obtained the g-C3N4/TiO2 nanosheets with high reactive (001) facets by a hydrothermal method, accompanied by a remarkable enhancement of photocatalytic capability in degradation of organic molecules under visible and UV light. Wang et al. successfully fabricated g-C3N4/BiPO4 g-C3N4 photocatalyst. The hybrid structure has been proved by Dong et al. to be a novel photocatalyst for the application of NO purification via an in situ deposition method [37]. Wang et al. explored the enhanced photocatalytic mechanism for the hybrid g-C3N4/MoS2 nanocomposites by DFT calculations. DFT calculations show these hybrid catalytic nanocomposites indeed have a higher absorption (used in the treatment of methylene orange (MO) [101]), with a decent photocatalytic performance and better separation of photo-generated carriers [38]. Ye’s group has demonstrated a zirconium-based metal–organic framework (Uio-66)-g-C3N4 nanosheet compound via a facile self-assembly method. Photocatalytic CO2 reduction activities were greatly enhanced. The photo-generated electron can be transferred from the g-C3N4 nanosheets to Uio-66 for better reduction of CO2 [48].
Ye’s group was inspired by the natural photosynthesis process and explored an environmental “phosphorylation” strategy to improve the photocatalytic performance [48]. A hydrogen generation rate of 947 µmol h−1 and a quantum yield of 26.1% at 425 nm were achieved. This is the highest record for g-C3N4-based photocatalyst [53]. Recently, Tong et al. were inspired by the function of thylakoids in a natural photosynthesis system as shown in Scheme 1a. They successfully fabricated a multishell g-C3N4 nanocapsule photocatalysts with enhanced light harvesting and electron transfer properties. The overall synthesis procedure is illustrated in Scheme 1b. The triple-shell g-C3N4 can produce hydrogen as much as 630 µmol h−1. This success potentially produces a new generation of solar devices for hydrogen production.
a Natural photosystem with green leafs, and the enlarged figure (right) depicts the light conversion in the stacked thylakoids. b Schematic illustration for the preparation of MSCN nanocapsules. Adapted with permission from Ref. [14]. Copyright 2017 American Chemical Society
Molecular doping not only plays an indispensable role in regulating the bandgap and electron structure of g-C3N4, but also controls the physical and chemical properties of g-C3N4. The bottom-up copolymerization method allows a large selection of structurally matched organic anchoring groups to be integrated into the g-C3N4 tri-triazine backbone to design highly efficient photocatalysts with the desired chemical composition and bandgap. It is expected that the modified g-C3N4 nanocrystals will provide an insightful view of the sustainable use of solar energy in chemistry because of the interesting new features that provide a new avenue for the study of heterogeneous catalysis.
g-C3N4 has a moderate bandgap of 2.7 eV, which makes it active in the visible region. Thermodynamic losses and overpotentials are usually considered in the photocatalytic process, the bandgap of 2.7 eV lying in between 2 eV (water splitting with enough endothermic driving forces) and 3.1 eV (visible-light absorption). What is more, g-C3N4 has a suitable conduction band position for various reduction reactions. The CB of g-C3N4 is more negative than H2-evolution, CO2-reduction, and O2-reduction reactions, revealing that the photo-generated electrons in g-C3N4 possess a large thermodynamic driving force to reduce small molecules like H2O, CO2, and O2. Therefore, the suitable electronic band structures of g-C3N4 are favorable for its applications as catalyst, such as water splitting, CO2 reduction, oxygen reduction reaction, oxygen evolution reaction, pollutant degradation, and organic synthesis.
Advantages and disadvantages Bandgap is crucial for photocatalytic applications. g-C3N4 has a direct moderate bandgap of 2.7 eV which has visible-light activity besides its advantages of low cost, metal-free, 2D layered structure, easy fabrication, and high stability. Unfortunately, the bulk g-C3N4 generally exhibits the low photocatalytic efficiency due to some serious drawbacks of g-C3N4 material, such as the high electron–hole recombination rate, low surface area, insufficient visible absorption, few active sites for interfacial reactions and low charge. Among various design strategies, heterojunction construction (especially for Z-scheme construction) and porosity design (microporous, mesoporous, and macroporous) have been employed. Based on present g-C3N4 materials, photocatalytic CO2 reduction seems to be more promising in developing practical g-C3N4-based photocatalysts.
4.2 g-C3N4 Sensing
It is well known that the polymeric g-C3N4 exhibits photoluminescence (PL) properties similar to many semiconductors materials. g-C3N4 emits a blue PL around 450 nm when dissolved in solvents under UV light irradiation due to its direct bandgap of 2.7 eV, which can be explained as the transition of the s-triazine ring [39]. Luminescent g-C3N4 (nanosheets and nanodots) can simply be regarded as nitrogen-rich carbon dots, although the quantum yield of g-C3N4 (up to 40%) is lower than carbon dots (up to 90%) [102]. g-C3N4 nanostructures exhibit a higher stability than the carbon dots, therefore potentially providing more practical applications. However, we are still far from the success of improving the quantum yield and understanding the precise PL mechanism of g-C3N4.
Based on the unique PL property of g-C3N4, g-C3N4 nanosheets have a strong response to copper ions [74] as turn-off chemical sensors. Since the chemical reduction of Cu2+ to Cu+ lies between the conduction band and valence band of g-C3N4, the PL of g-C3N4 can be quenched with a low detection limit of 0.5 nM [103]. A similar mechanism has been explained in previous work [104]. In that work, a relatively low detection limit of 0.04 nM has been achieved by CdTe QDs. Besides copper ions, PL of g-C3N4 can also be quenched by other metal ions like Fe3+, Ag+, Hg2+, and Cr2+ [89, 90, 105,106,107,108,109,110]. Huang et al. reported the fabrication of g-C3N4 nanosheets for the selective detection to Cu2+ and Ag+. Zhang et al. successfully prepared the g-C3N4 QDs as effective fluorescent probes for the detection of Fe3+ and Cu2+. Cao et al. developed the g-C3N4 nanodots via a microwave-assisted approach. The produced nanodots were utilized as turn-off sensors for mercury ions with a detection limit of 0.14 μM [89]. Shao’s group successfully synthesized the oxygen- and sulfur-co-doped g-C3N4 nanodots via a hydrothermal method, which has a lower detection limit of mercury ions (0.37 nM) [90]. Sun’s group successfully fabricated Fe-doped g-C3N4 nanosheets with a highly sensitive optical detection of glucose due to the chelation of Fe3+ with N [123]. Moreover, they demonstrated the ultrathin g-C3N4 nanosheets can be utilized as electrochemical glucose biosensing with a detection limit of 11 μM [124]. Rong et al. explored turn-off–turn-on sensors using g-C3N4 nanosheets, Cr and ascorbic acid. After Cr quenches the PL signal of g-C3N4, the addition of ascorbic acid can recover the PL signal due to the oxidation–reduction between Cr and ascorbic acid. Yang’s group fabricated a g-C3N4 nanosheet/MnO2 sandwich nanocomposite via a one-step approach. The fabricated composites could turn-on the fluorescent probes of glutathione (GSH) with a high selectivity due to fluorescence resonance energy transfer (FRET), as shown in Scheme 2a [111]. GSH is possibly a suitable recovering agent of the PL signal of g-C3N4 as well. Xu et al. reported a g-C3N4/Hg system without PL signal, which can be switched on by an introduction of GSH. The system may be employed for detection of GSH in various food samples [112]. Based on FRET, g-C3N4 was found to detect riboflavin (RF) because g-C3N4 acts as a donor of energy transfers and RF as an acceptor [113]. A turn-on g-C3N4-based long-persistent luminescent probe for detection of biothiols was reported by Tang et al. [115] for the first time. This long-persistent luminescence allows the detection without external illumination. Qiao’s group has successfully prepared the proton-functionalized ultrathin g-C3N4 nanosheets which can interact with heparin, therefore achieving the lowest detection limit of 18 ng mL−1 [52].
a Schematic representation of g-C3N4/MnO2 nanocomposite for sensing of GSH. Reproduced with permission from Ref. [107]. Copyright 2014 American Chemical Society. b Schematic illustration of the dual-wavelength ratiometric ECL-RET biosensor configuration strategy. Reproduced with permission from Ref. [114]. Copyright 2016 American Chemical Society
Feng et al. successfully fabricated Au-nanoparticle-functionalized g-C3N4 nanosheets coupled with Ru(bpy) 2+3 . The coupled g-C3N4 nanosheets can be employed for RNA detection based on dual-wavelength electrochemiluminescence (ECL), as shown in Scheme 2b. Au/g-C3N4 composites exhibit an emission at 460 nm and g-C3N4/Ru(bpy) 2+3 at 620 nm. Here, g-C3N4 acts as a donor of energy transfer and Ru(bpy) 2+3 as an acceptor for highly sensitive and selective detection of target miRNA (ECL signals quenching at 460 nm and increasing at 620 nm) [114].
In addition to detecting metal ions and bio-molecules by g-C3N4, g-C3N4 can also respond to temperature [116]. Debanjan et al. reported a temperature sensor based on the PL of g-C3N4. They found that as the temperature increased, the intensity of PL decreased.
4.3 g-C3N4 Imaging
Non-toxicity, metal-free, high stability, and high PL quantum yield enable g-C3N4 nanosheets and nanodots to be promising candidates for cell imaging. Xie’s group demonstrated the preparation of ultrathin g-C3N4 nanosheets for bio-imaging applications [65]. They found that g-C3N4 nanosheets have no significant effects on the HeLa cell viability even at a high concentration. The same group further developed the single-layered g-C3N4 QDs for both one-photon and two-photon cell imaging as long as the QDs can pass through the nuclear pore and enter into the nuclei [87]. Singlet oxygen, being one of the most important reactive oxygen species (ROS), could be generated in the presence of g-C3N4 as a photosensitizer [95].
Recently, Yang’s group reported the NIR-driven g-C3N4/up-conversion nanoparticle (UCNP) composite for efficient bio-imaging and photodynamic therapy (PDT) [117]. In this application, g-C3N4 functions as a sensitizer to absorb the UV light converted by UCNPs from 980 nm NIR light. The generated ROS causes the tumor to shrink or disappear effectively without any side effects from the irradiation [117]. However, long-time irradiation of 980 nm NIR light can cause overheating of tissues; therefore, an 808 nm laser light is more suitable for the PDT.
Feng et al. [118] fabricated a novel core–shell structure (UCNP/g-C3N4) for phototherapy and imaging applications. Mesoporous g-C3N4 was coated outside of the shell of UCNPs, generating a large amount of ROS due to the large surface area of g-C3N4 under the 808 nm laser irradiation. In vivo experiments were conducted as shown in Fig. 9. The factors affecting the efficiency of sensing and imaging are mainly the quantum yield, functionalization, PL and optical properties, stability, and toxicity.
a In vivo tumor volume growth curves of mice in different groups after various treatments. b Body weight changes of Balb/c mice versus treated time under different conditions. c Photographs of excised tumors from representative Balb/c mice after 14 day treatment and d the corresponding digital photographs of mice in the control group and “UCNPs@g-C3N4 − PEG with 808 nm laser” group after 14 day treatment. e H&E stained tumor sections after 14 day treatment from different groups. Reproduced with permission from Ref. [118] Copyright 2016 American Chemical Society
4.4 g-C3N4-Based LED
Although the PL properties of g-C3N4 have been investigated in the past fifteen years, the solid-state lighting of g-C3N4-based materials is still at an infancy stage. Various investigations about the g-C3N4-based solid-state lighting such as white-light-emitting diodes (WLEDs) have been carried out. Wang et al. fabricated the g-C3N4/silica gels for WLEDs application (Fig. 10a–d). In this work, using a one-step heat treatment approach confirmed by the FTIR, g-C3N4 was found to be covalently bonded with silica gels (Fig. 10e, f). The g-C3N4/silica gels obtained possess emerging properties with respect to bare g-C3N4, including water-resistance, high transparency, high flexibility, and white light emission under UV irradiation. The mechanism for white light emission can be ascribed to surface passivation by silica [119]. Bayan et al. [120] prepared a g-C3N4 sheet/ZnO nanorod hybrid for WLEDs by combining the emissions of g-C3N4 and ZnO nanorods to achieve a broad emission. Last but not the least, Gan et al. studied the origins of broad PL of g-C3N4 for the WLEDs applications. They demonstrated that the broad PL from g-C3N4 is attributed to band-to-band transitions in the tri-s-triazine rings. This novel work can help the understanding of the PL mechanism and accelerate the progress of WLEDs-based g-C3N4.
a Photoluminescence spectrum of the g-C3N4/silica gels excited at 365 nm displaying four peaks (430, 480, 580, and 627 nm) in the visible regime. b, c Photographs of a free-standing g-C3N4/silica-gel membrane, displaying both good transparency b and flexibility c. d CIE-1931 chromaticity diagram showing the emission from the typical g-C3N4/silica gels (marked by the black cross) excited at 365 nm. e FTIR spectra of AEATMS, g-C3N4-silica gels, and g-C3N4 particles. f Schematic drawing of an AEATMS-capped g-C3N4 particles in the g-C3N4/silica gels. Reproduced with permission from Ref. [119]. Copyright 2016 Wiley
5 Conclusion and Outlook
This review summarizes the recent advances of g-C3N4-based structures and applications including catalyst, chemical and biosensing, imaging, and LEDs. The performances of g-C3N4 are mainly based on their surface state (defects, function groups, and doping) and structures (porosity, thickness, and morphology). Although a significant advancement has been made for the development of highly efficient g-C3N4-based photocatalysts, there are still considerable problems that require further investigations, including the catalytic rate and design.
2D polymeric g-C3N4 materials featuring low cost, metal-free, environmental friendly, moderate bandgap, high chemistry activity, and high stability have only been studied for the past few years (from fundamental research to practical applications). We believe that more emerging properties and applications of g-C3N4 are around the corner. Integrations between experimental research and theoretical approaches will advance the research progress of g-C3N4 to a large extent. As the exploration of g-C3N4 that is still in its infancy, there are several remaining key challenges that must be met in near future, including porous nanostructures for the drug loading and delivery, improvement of electronic conductivity, memory device fabrication, solid-state lighting, energy conversion, and wearable sensors.
References
J. Von, Liebig, Uber einige stickstoff-verbindungen. Eur. J. Organic Chem. 10(1), 1–47 (1834). doi:10.1002/jlac.18340100102
A.Y. Liu, M.L. Cohen, Prediction of new low compressibility solids. Science 245(4920), 841–842 (1989). doi:10.1126/science.245.4920.841
F. Goettmann, A. Fischer, M. Antonietti, A. Thomas, Metal-free catalysis of sustainable Friedel–Crafts reactions: direct activation of benzene by carbon nitrides to avoid the use of metal chlorides and halogenated compounds. Chem. Commun. 43, 4530–4532 (2006). doi:10.1039/B608532F
D.M. Teter, R.J. Hemley, Low-compressibility carbon nitrides. Science 271(5245), 53–55 (1996). doi:10.1126/science.271.5245.53
X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J.M. Carlsson, K. Domen, M. Antonietti, A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 8(1), 76–80 (2009). doi:10.1038/NMAT2317
Y.S. Jun, W.H. Hong, M. Antonietti, A. Thomas, Mesoporous, 2D hexagonal carbon nitride and titanium nitride/carbon composites. Adv. Mater. 21(42), 4270–4274 (2009). doi:10.1002/adma.200803500
E. Kroke, M. Schwarz, E. Horath-Bordon, P. Kroll, B. Noll, A.D. Norman, Tri-s-triazine derivatives. Part I. From trichloro-tri-s-triazine to graphitic C3N4 structures. New J. Chem. 26(5), 508–512 (2002). doi:10.1039/b111062b
A. Fujishima, Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). doi:10.1038/238037a0
H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri, J. Ye, Nano-photocatalytic materials: possibilities and challenges. Adv. Mater. 24(2), 229–251 (2012). doi:10.1002/adma.201102752
Y. Zheng, L. Fu, F. Han, A. Wang, W. Cai, J. Yu, J. Yang, F. Peng, Green biosynthesis and characterization of zinc oxide nanoparticles using Corymbia citriodora leaf extract and their photocatalytic activity. Green Chem. Lett. Rev. 8(2), 59–63 (2015). doi:10.1080/17518253.2015.1075069
C. Wang, A.W. Wang, J. Feng, Z. Li, B. Chen, Q.-H. Wu, J. Jiang, J. Lu, Y.Y. Li, Hydrothermal preparation of hierarchical MoS2-reduced graphene oxide nanocomposites towards remarkable enhanced visible-light photocatalytic activity. Ceram. Int. 43(2), 2384–2388 (2017). doi:10.1016/j.matlet.2014.12.021
L. Fu, W. Cai, A. Wang, Y. Zheng, Photocatalytic hydrogenation of nitrobenzene to aniline over tungsten oxide-silver nanowires. Mater. Lett. 142, 201–203 (2015). doi:10.1016/j.matlet.2014.12.021
Y. Hou, Z. Wen, S. Cui, X. Feng, J. Chen, Strongly coupled ternary hybrid aerogels of N-deficient porous graphitic-C3N4 nanosheets/N-doped graphene/NiFe-layered double hydroxide for solar-driven photoelectrochemical water oxidation. Nano Lett. 16(4), 2268–2277 (2016). doi:10.1021/acs.nanolett.5b04496
Z. Tong, D. Yang, Z. Li, Y. Nan, F. Ding, Y. Shen, Z. Jiang, Thylakoid-inspired multi-shell g-C3N4 nanocapsules with enhanced visible-light harvesting and electron transfer properties for high-efficiency photocatalysis. ACS Nano 11(1), 1103–1112 (2017). doi:10.1021/acsnano.6b08251
G. Wu, Y. Hu, Y. Liu, J. Zhao, X. Chen, V. Whoehling, C. Plesse, G.T. Nguyen, F. Vidal, W. Chen, Graphitic carbon nitride nanosheet electrode-based high-performance ionic actuator. Nat. Commun. 6, 7258 (2015). doi:10.1038/ncomms8258
C. Li, Y. Du, D. Wang, S. Yin, W. Tu, Z. Chen, M. Kraft, G. Chen, R. Xu, Unique P-Co-N surface bonding states constructed on g-C3N4 nanosheets for drastically enhanced photocatalytic activity of H2 evolution. Adv. Funct. Mater. 27(4), 1604328 (2016). doi:10.1002/adfm.201604328
X. Liu, L. Dai, Carbon-based metal-free catalysts. Nat. Rev. Mater. 1, 16064 (2016). doi:10.1038/natrevmats.2016.64
Y. Zheng, Y. Jiao, Y. Zhu, L.H. Li, Y. Han, Y. Chen, A. Du, M. Jaroniec, S.Z. Qiao, Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 5, 3783 (2014). doi:10.1038/ncomms4783
Q. Guo, Y. Zhang, J. Qiu, G. Dong, Engineering the electronic structure and optical properties of g-C3N4 by non-metal ion doping. J. Mater. Chem. C 4(28), 6839–6847 (2016). doi:10.1039/c6tc01831a
F. He, G. Chen, Y. Yu, Y. Zhou, Y. Zheng, S. Hao, The synthesis of condensed C-PDA-C3N4 composites with superior photocatalytic performance. Chem. Commun. 51(31), 6824–6827 (2015). doi:10.1039/c5cc01013f
Y. Li, S. Ouyang, H. Xu, X. Wang, Y. Bi, Y. Zhang, J. Ye, Constructing solid–gas-interfacial fenton reaction over alkalinized-C3N4 photocatalyst to achieve apparent quantum yield of 49% at 420 nm. J. Am. Chem. Soc. 138(40), 13289–13297 (2016). doi:10.1021/jacs.6b07272
S. Kumar, B. Kumar, A. Baruah, V. Shanker, Synthesis of magnetically separable and recyclable g-C3N4–Fe3O4 hybrid nanocomposites with enhanced photocatalytic performance under visible-light irradiation. J. Phys. Chem. 117(49), 26135–26143 (2013). doi:10.1021/jp409651g
J. Zhang, M. Zhang, L. Lin, X. Wang, Sol processing of conjugated carbon nitride powders for thin-film fabrication. Angew. Chem. Int. Ed. 54(21), 6297–6301 (2015). doi:10.1002/anie.201501001
J. Bian, Q. Li, C. Huang, J. Li, Y. Guo, M. Zaw, R.-Q. Zhang, Thermal vapor condensation of uniform graphitic carbon nitride films with remarkable photocurrent density for photoelectrochemical applications. Nano Energy 15, 353–361 (2015). doi:10.1016/j.nanoen.2015.04.012
Y. Feng, J. Shen, Q. Cai, H. Yang, Q. Shen, The preparation and properties of a gC3N4/AgBr nanocomposite photocatalyst based on protonation pretreatment. New J. Chem. 39(2), 1132–1138 (2015). doi:10.1039/C4NJ01433B
M. Shalom, S. Gimenez, F. Schipper, I. Herraiz-Cardona, J. Bisquert, M. Antonietti, Controlled carbon nitride growth on surfaces for hydrogen evolution electrodes. Angew. Chem. Int. Ed. 126(14), 3728–3732 (2014). doi:10.1002/ange.201309415
P. Wu, J. Wang, J. Zhao, L. Guo, F.E. Osterloh, Structure defects in g-C3N4 limit visible light driven hydrogen evolution and photovoltage. J. Mater. Chem. A 2(47), 20338–20344 (2014). doi:10.1039/c4ta04100c
K. Srinivasu, B. Modak, S.K. Ghosh, Porous graphitic carbon nitride: a possible metal-free photocatalyst for water splitting. J. Phys. Chem. C 118(46), 26479–26484 (2014). doi:10.1021/jp506538d
J. Duan, S. Chen, M. Jaroniec, S.Z. Qiao, Porous C3N4 nanolayers@ N-graphene films as catalyst electrodes for highly efficient hydrogen evolution. ACS Nano 9(1), 931–940 (2015). doi:10.1021/nn506701x
M. Tahir, C. Cao, N. Mahmood, F.K. Butt, A. Mahmood et al., Multifunctional g-C3N4 nanofibers: a template-free fabrication and enhanced optical, electrochemical, and photocatalyst properties. ACS Appl. Mater. Interfaces 6(2), 1258–1265 (2013). doi:10.1021/am405076b
Y. Guo, F. Kong, C. Wang, S. Chu, J. Yang, Y. Wang, Z. Zou, Molecule-induced gradient electronic potential distribution on a polymeric photocatalyst surface and improved photocatalytic performance. J. Mater. Chem. A 1(16), 5142–5147 (2013). doi:10.1039/c3ta10528h
J. Liu, H. Wang, Z.P. Chen, H. Moehwald, S. Fiechter, R. van de Krol, L. Wen, L. Jiang, M. Antonietti, Microcontact-printing-assisted access of graphitic carbon nitride films with favorable textures toward photoelectrochemical application. Adv. Mater. 27(4), 712–718 (2015). doi:10.1002/adma.201404543
Y.-P. Zhu, T.-Z. Ren, Z.-Y. Yuan, Mesoporous phosphorus-doped g-C3N4 nanostructured flowers with superior photocatalytic hydrogen evolution performance. ACS Appl. Mater. Interfaces 7(30), 16850–16856 (2015). doi:10.1021/acsami.5b04947
Y. Kang, Y. Yang, L.C. Yin, X. Kang, G. Liu, H.M. Cheng, An amorphous carbon nitride photocatalyst with greatly extended visible-light-responsive range for photocatalytic hydrogen generation. Adv. Mater. 27(31), 4572–4577 (2015). doi:10.1002/adma.201501939
Y. Shiraishi, S. Kanazawa, Y. Sugano, D. Tsukamoto, H. Sakamoto, S. Ichikawa, T. Hirai, Highly selective production of hydrogen peroxide on graphitic carbon nitride (g-C3N4) photocatalyst activated by visible light. ACS Catal. 4(3), 774–780 (2014). doi:10.1021/cs401208c
Y. Chen, W. Huang, D. He, Y. Situ, H. Huang, Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Interfaces 6(16), 14405–14414 (2014). doi:10.1021/am503674e
F. Dong, Z. Zhao, Y. Sun, Y. Zhang, S. Yan, Z. Wu, An advanced semimetal–organic bi spheres–g-C3N4 nanohybrid with SPR-enhanced visible-light photocatalytic performance for NO purification. Environ. Sci. Technol. 49(20), 12432–12440 (2015). doi:10.1021/acs.est.5b03758
J. Wang, Z. Guan, J. Huang, Q. Li, J. Yang, Enhanced photocatalytic mechanism for the hybrid g-C3N4/MoS2 nanocomposite. J. Mater. Chem. A 2(21), 7960–7966 (2014). doi:10.1039/c4ta00275j
A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J.-O. Müller, R. Schlögl, J.M. Carlsson, Graphitic carbon nitride materials: variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 18(41), 4893–4908 (2008). doi:10.1039/b800274f
W.-J. Ong, L.-L. Tan, Y.H. Ng, S.-T. Yong, S.-P. Chai, Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev. 116(12), 7159–7329 (2016). doi:10.1021/acs.chemrev.6b00075
Z. Zhao, Y. Sun, F. Dong, Graphitic carbon nitride based nanocomposites: a review. Nanoscale 7(1), 15–37 (2015). doi:10.1039/C4NR03008G
J. Zhu, P. Xiao, H. Li, S.A. Carabineiro, Graphitic carbon nitride: synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 6(19), 16449–16465 (2014). doi:10.1021/am502925j
L. Zhou, H. Zhang, H. Sun, S. Liu, M.O. Tade, S. Wang, W. Jin, Recent advances in non-metal modification of graphitic carbon nitride for photocatalysis: a historic review. Catal. Sci. Technol. 6(19), 7002–7023 (2016). doi:10.1039/C6CY01195K
Y. Zheng, L. Lin, B. Wang, X. Wang, Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Int. Ed. 54(44), 12868–12884 (2015). doi:10.1002/anie.201501788
X.H. Li, M. Antonietti, Metal nanoparticles at mesoporous N-doped carbons and carbon nitrides: functional Mott-Schottky heterojunctions for catalysis. Chem. Soc. Rev. 42(16), 6593–6604 (2013). doi:10.1039/c3cs60067j
J. Liu, W. Li, L. Duan, X. Li, L. Ji et al., A Graphene-like oxygenated carbon nitride material for improved cycle-life lithium/sulfur batteries. Nano Lett. 15(8), 5137–5142 (2015). doi:10.1021/acs.nanolett.5b01919
G.S. Li, Z.C. Lian, W.C. Wang, D.Q. Zhang, H.X. Li, Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy 19, 446–454 (2016). doi:10.1016/j.nanoen.2015.10.011
L. Shi, T. Wang, H. Zhang, K. Chang, J. Ye, Electrostatic self-assembly of nanosized carbon nitride nanosheet onto a zirconium metal-organic framework for enhanced photocatalytic CO2 reduction. Adv. Funct. Mater. 25(33), 5360–5367 (2015). doi:10.1002/adfm.201502253
J.W. Fang, H.Q. Fan, M.M. Li, C.B. Long, Nitrogen self-doped graphitic carbon nitride as efficient visible light photocatalyst for hydrogen evolution. J. Mater. Chem. A 3(26), 13819–13826 (2015). doi:10.1039/c5ta02257f
G. Zhang, M. Zhang, X. Ye, X. Qiu, S. Lin, X. Wang, Iodine modified carbon nitride semiconductors as visible light photocatalysts for hydrogen evolution. Adv. Mater. 26(5), 805–809 (2014). doi:10.1002/adma.201303611
Q.H. Liang, Z. Li, Z.H. Huang, F.Y. Kang, Q.H. Yang, Holey graphitic carbon nitride nanosheets with carbon vacancies for highly improved photocatalytic hydrogen production. Adv. Funct. Mater. 25(44), 6885–6892 (2015). doi:10.1002/adfm.201503221
T.Y. Ma, Y. Tang, S. Dai, S.Z. Qiao, Proton-functionalized two-dimensional graphitic carbon nitride nanosheet: an excellent metal-/label-free biosensing platform. Small 10(12), 2382–2389 (2014). doi:10.1002/smll.201303827
G. Liu, T. Wang, H. Zhang, X. Meng, D. Hao, K. Chang, P. Li, T. Kako, J. Ye, Nature-inspired environmental phosphorylation boosts photocatalytic H2 production over carbon nitride nanosheets under visible-light irradiation. Angew. Chem. Int. Ed. 127(46), 13765–13769 (2015). doi:10.1002/ange.201505802
X.D. Sun, Y.Y. Li, J. Zhou, C. Hai Ma, Y. Wang, J.H. Zhu, Facile synthesis of high photocatalytic active porous g-C3N4 with ZnCl2 template. J. Colloid Interface Sci. 451, 108–116 (2015). doi:10.1016/j.jcis.2015.03.059
J. Zhu, Y. Wei, W. Chen, Z. Zhao, A. Thomas, Graphitic carbon nitride as a metal-free catalyst for NO decomposition. Chem. Commun. 46(37), 6965–6967 (2010). doi:10.1039/c0cc01432j
Y. Shiraishi, Y. Kofuji, H. Sakamoto, S. Tanaka, S. Ichikawa, T. Hirai, Effects of surface defects on photocatalytic H2O2 production by mesoporous graphitic carbon nitride under visible light irradiation. ACS Catal. 5(5), 3058–3066 (2015). doi:10.1021/acscatal.5b00408
J. Xiao, Y. Xie, F. Nawaz, Y. Wang, P. Du, H. Cao, Dramatic coupling of visible light with ozone on honeycomb-like porous g-C3N4 towards superior oxidation of water pollutants. Appl. Catal. B 183, 417–425 (2016). doi:10.1016/j.apcatb.2015.11.010
K. Wang, Q. Li, B. Liu, B. Cheng, W. Ho, J. Yu, Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B 176, 44–52 (2015). doi:10.1016/j.apcatb.2015.03.045
J. Xu, H.T. Wu, X. Wang, B. Xue, Y.X. Li, Y. Cao, A new and environmentally benign precursor for the synthesis of mesoporous g-C3N4 with tunable surface area. Phys. Chem. Chem. Phys. 15(13), 4510–4517 (2013). doi:10.1039/c3cp44402c
Y.J. Chung, B.I. Lee, J.W. Ko, C.B. Park, Photoactive g-C3N4 nanosheets for light-induced suppression of alzheimer’s beta-amyloid aggregation and toxicity. Adv. Healthc. Mater. 5(13), 1560–1565 (2016). doi:10.1002/adhm.201500964
W. Shan, Y. Hu, Z. Bai, M. Zheng, C. Wei, In situ preparation of g-C3N4/bismuth-based oxide nanocomposites with enhanced photocatalytic activity. Appl. Catal. B 188, 1–12 (2016). doi:10.1016/j.apcatb.2016.01.058
M. Sevilla, R. Mokaya, Energy storage applications of activated carbons: supercapacitors and hydrogen storage. Energy Environ. Sci. 7(4), 1250–1280 (2014). doi:10.1039/c3ee43525c
C.Y. Liu, Y.H. Zhang, F. Dong, X. Du, H.W. Huang, Easily and synchronously ameliorating charge separation and band energy level in porous g-C3N4 for boosting photooxidation and photoreduction ability. J. Phys. Chem. C 120(19), 10381–10389 (2016). doi:10.1021/acs.jpcc.6b01705
S. Pandiaraj, H.B. Aiyappa, R. Banerjee, S. Kurungot, Post modification of MOF derived carbon via g-C3N4 entrapment for an efficient metal-free oxygen reduction reaction. Chem. Commun. 50(25), 3363–3366 (2014). doi:10.1039/c3cc47620k
X. Zhang, X. Xie, H. Wang, J. Zhang, B. Pan, Y. Xie, Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 135(1), 18–21 (2013). doi:10.1021/ja308249k
K. Chen, Z. Chai, C. Li, L. Shi, M. Liu, Q. Xie, Y. Zhang, D. Xu, A. Manivannan, Z. Liu, Catalyst-free growth of three-dimensional graphene flakes and graphene/g-C(3)N(4) composite for hydrocarbon oxidation. ACS Nano 10(3), 3665–3673 (2016). doi:10.1021/acsnano.6b00113
X. Yuan, C. Zhou, Y. Jin, Q. Jing, Y. Yang, X. Shen, Q. Tang, Y. Mu, A.K. Du, Facile synthesis of 3D porous thermally exfoliated g-C3N4 nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interface Sci. 468, 211–219 (2016). doi:10.1016/j.jcis.2016.01.048
J.S. Zhang, Y. Chen, X.C. Wang, Two-dimensional covalent carbon nitride nanosheets: synthesis, functionalization, and applications. Energy Environ. Sci. 8(11), 3092–3108 (2015). doi:10.1039/c5ee01895a
K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, A.A. Firsov, Electric field effect in atomically thin carbon films. Science 306(5696), 666–669 (2004). doi:10.1126/science.1102896
C. Wang, Y. Zhou, L. He, T.W. Ng, G. Hong, Q.H. Wu, F. Gao, C.S. Lee, W. Zhang, In situ nitrogen-doped graphene grown from polydimethylsiloxane by plasma enhanced chemical vapor deposition. Nanoscale 5(2), 600–605 (2013). doi:10.1039/c2nr32897f
C.D. Wang, Y.S. Chui, R.G. Ma, T.L. Wong, J.G. Ren, Q.H. Wu, X.F. Chen, W.J. Zhang, A three-dimensional graphene scaffold supported thin film silicon anode for lithium-ion batteries. J. Mater. Chem. A 1(35), 10092–10098 (2013). doi:10.1039/c3ta11740e
C.D. Wang, J.L. Xu, M.F. Yuen, J. Zhang, Y.Y. Li, X.F. Chen, W.J. Zhang, Hierarchical composite electrodes of nickel oxide nanoflake 3D graphene for high-performance pseudocapacitors. Adv. Funct. Mater. 24(40), 6372–6380 (2014). doi:10.1002/adfm.201401216
Q. Han, F. Zhao, C. Hu, L. Lv, Z. Zhang, N. Chen, L. Qu, Facile production of ultrathin graphitic carbon nitride nanoplatelets for efficient visible-light water splitting. Nano Res. 8(5), 1718–1728 (2015). doi:10.1007/s12274-014-0675-9
N. Cheng, P. Jiang, Q. Liu, J. Tian, A.M. Asiri, X. Sun, Graphitic carbon nitride nanosheets: one-step, high-yield synthesis and application for Cu2+ detection. Analyst 139(20), 5065–5068 (2014). doi:10.1039/c4an00914b
P. Niu, L.L. Zhang, G. Liu, H.M. Cheng, Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 22(22), 4763–4770 (2012). doi:10.1002/adfm.201200922
K. Schwinghammer, M.B. Mesch, V. Duppel, C. Ziegler, J. Senker, B.V. Lotsch, Crystalline carbon nitride nanosheets for improved visible-light hydrogen evolution. J. Am. Chem. Soc. 136(5), 1730–1733 (2014). doi:10.1021/ja411321s
J.S. Zhang, J.H. Sun, K. Maeda, K. Domen, P. Liu, M. Antonietti, X.Z. Fu, X.C. Wang, Sulfur-mediated synthesis of carbon nitride: band-gap engineering and improved functions for photocatalysis. Energy Environ. Sci. 4(3), 675–678 (2011). doi:10.1039/c0ee00418a
S. Yang, X. Feng, X. Wang, K. Mullen, Graphene-based carbon nitride nanosheets as efficient metal-free electrocatalysts for oxygen reduction reactions. Angew. Chem. Int. Ed. 50(23), 5339–5343 (2011). doi:10.1002/anie.201100170
S. Wang, C. Li, T. Wang, P. Zhang, A. Li, J. Gong, Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J. Mater. Chem. A 2(9), 2885–2890 (2014). doi:10.1039/c3ta14576j
Y. Zheng, L. Lin, X. Ye, F. Guo, X. Wang, Helical graphitic carbon nitrides with photocatalytic and optical activities. Angew. Chem. Int. Ed. 53(44), 11926–11930 (2014). doi:10.1002/anie.201407319
J. Liu, J. Huang, H. Zhou, M. Antonietti, Uniform graphitic carbon nitride nanorod for efficient photocatalytic hydrogen evolution and sustained photoenzymatic catalysis. ACS Appl. Mater. Interfaces 6(11), 8434–8440 (2014). doi:10.1021/am501319v
X.-H. Li, X. Wang, M. Antonietti, Mesoporous g-C3N4 nanorods as multifunctional supports of ultrafine metal nanoparticles: hydrogen generation from water and reduction of nitrophenol with tandem catalysis in one step. Chem. Sci. 3(6), 2170–2174 (2012). doi:10.1039/c2sc20289a
F. He, G. Chen, J. Miao, Z. Wang, D. Su et al., Sulfur-mediated self-templating synthesis of tapered C-PAN/g-C3N4 composite nanotubes toward efficient photocatalytic H2 evolution. ACS Energy Lett. 1(5), 969–975 (2016). doi:10.1021/acsenergylett.6b00398
S. Panneri, M. Thomas, P. Ganguly, B.N. Nair, A.M. Peer, K.G.K. Warrier, H.U.N. Saraswathy, C3N4 anchored ZIF 8 composites: photo-regenerable, high capacity sorbents as adsorptive photocatalysts for the effective removal of tetracycline from water. Catal. Sci. Technol. 7, 2118–2128 (2017). doi:10.1039/C7CY00348J
Z. Tong, D. Yang, Y. Sun, Y. Nan, Z. Jiang, Tubular g-C3N4 isotype heterojunction: enhanced visible-light photocatalytic activity through cooperative manipulation of oriented electron and hole transfer. Small 12(30), 4093–4101 (2016). doi:10.1002/smll.201601660
W. Wang, C.Y. Jimmy, Z. Shen, D.K. Chan, T. Gu, g-C3N4 quantum dots: direct synthesis, upconversion properties and photocatalytic application. Chem. Commun. 50(70), 10148–10150 (2014). doi:10.1039/c4cc02543a
X.D. Zhang, H.X. Wang, H. Wang, Q. Zhang, J.F. Xie, Y.P. Tian, J. Wang, Y. Xie, Single-layered graphitic-C3N4 quantum dots for two-photon fluorescence imaging of cellular nucleus. Adv. Mater. 26(26), 4438–4443 (2014). doi:10.1002/adma.201400111
J. Wu, S. Yang, J. Li, Y. Yang, G. Wang et al., Electron injection of phosphorus doped g-C3N4 quantum dots: controllable photoluminescence emission wavelength in the whole visible light range with high quantum yield. Adv. Opt. Mater. 4(12), 2095–2101 (2016). doi:10.1002/adom.201600570
X. Cao, J. Ma, Y. Lin, B. Yao, F. Li, W. Weng, X. Lin, A facile microwave-assisted fabrication of fluorescent carbon nitride quantum dots and their application in the detection of mercury ions. Spectrochim. Acta A 151, 875–880 (2015). doi:10.1016/j.saa.2015.07.034
Y.-C. Lu, J. Chen, A.-J. Wang, N. Bao, J.-J. Feng, W. Wang, L. Shao, Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury (II) detection and bioimaging. J. Mater. Chem. C 3(1), 73–78 (2015). doi:10.1039/c4tc02111h
P. Niu, L.C. Yin, Y.Q. Yang, G. Liu, H.M. Cheng, Increasing the visible light absorption of graphitic carbon nitride (melon) photocatalysts by homogeneous self-modification with nitrogen vacancies. Adv. Mater. 26(47), 8046–8052 (2014). doi:10.1002/adma.201404057
J. Sun, Y. Fu, G. He, X. Sun, X. Wang, Green Suzuki-Miyaura coupling reaction catalyzed by palladium nanoparticles supported on graphitic carbon nitride. Appl. Catal. B 165, 661–667 (2015). doi:10.1016/j.apcatb.2014.10.072
A. Kumar, P. Kumar, C. Joshi, S. Ponnada, A.K. Pathak, A. Ali, B. Sreedhar, S.L. Jain, A [Fe(bpy)(3)](2 +) grafted graphitic carbon nitride hybrid for visible light assisted oxidative coupling of benzylamines under mild reaction conditions. Green Chem. 18(8), 2514–2521 (2016). doi:10.1039/c5gc02090e
P. Sharma, Y. Sasson, A photoactive catalyst Ru–g-C3N4 for hydrogen transfer reaction of aldehydes and ketones. Green Chem. 19, 844–852 (2017). doi:10.1039/c6gc02949c
H. Wang, S. Jiang, S. Chen, D. Li, X. Zhang et al., Enhanced singlet oxygen generation in oxidized graphitic carbon nitride for organic synthesis. Adv. Mater. 28(32), 6940–6945 (2016). doi:10.1002/adma.201601413
M. Zhang, X. Wang, Two dimensional conjugated polymers with enhanced optical absorption and charge separation for photocatalytic hydrogen evolution. Energy Environ. Sci. 7(6), 1902–1906 (2014). doi:10.1039/C3EE44189J
J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang et al., Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347(6225), 970–974 (2015). doi:10.1126/science.aaa3145
K. Dai, L. Lu, Q. Liu, G. Zhu, X. Wei, J. Bai, L. Xuan, H. Wang, Sonication assisted preparation of graphene oxide/graphitic-C3N4 nanosheet hybrid with reinforced photocurrent for photocatalyst applications. Dalton Trans. 43(17), 6295–6299 (2014). doi:10.1039/c3dt53106f
Z. Ma, R. Sa, Q. Li, K. Wu, Interfacial electronic structure and charge transfer of hybrid graphene quantum dot and graphitic carbon nitride nanocomposites: insights into high efficiency for photocatalytic solar water splitting. Phys. Chem. Chem. Phys. 18(2), 1050–1058 (2016). doi:10.1039/c5cp05847c
L. Gu, J. Wang, Z. Zou, X. Han, Graphitic-C3N4-hybridized TiO2 nanosheets with reactive 001 facets to enhance the UV-and visible-light photocatalytic activity. J. Hazard. Mater. 268, 216–223 (2014). doi:10.1016/j.jhazmat.2014.01.021
Z. Li, B. Li, S. Peng, D. Li, S. Yang, Y. Fang, Novel visible light-induced g-C3N4 quantum dot/BiPO4 nanocrystal composite photocatalysts for efficient degradation of methyl orange. RSC Adv. 4(66), 35144–35148 (2014). doi:10.1039/c4ra05749j
S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song et al., Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 52(14), 3953–3957 (2013). doi:10.1002/anie.201300519
J. Tian, Q. Liu, A.M. Asiri, A.O. Al-Youbi, X. Sun, Ultrathin graphitic carbon nitride nanosheet: a highly efficient fluorosensor for rapid, ultrasensitive detection of Cu(2+). Anal. Chem. 85(11), 5595–5599 (2013). doi:10.1021/ac400924j
A. Wang, L. Fu, T. Rao, W. Cai, M.-F. Yuen, J. Zhong, Effect of metal ions on the quenching of photoluminescent CdTe QDs and their recovery. Opt. Mater. 42, 548–552 (2015). doi:10.1016/j.optmat.2015.01.010
S. Zhang, J. Li, M. Zeng, J. Xu, X. Wang, W. Hu, Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nanoscale 6(8), 4157–4162 (2014). doi:10.1039/C3NR06744K
M. Rong, L. Lin, X. Song, Y. Wang, Y. Zhong, J. Yan, Y. Feng, X. Zeng, X. Chen, Fluorescence sensing of chromium (VI) and ascorbic acid using graphitic carbon nitride nanosheets as a fluorescent switch. Biosens. Bioelectron. 68, 210–217 (2015). doi:10.1016/j.bios.2014.12.024
H. Huang, R. Chen, J. Ma, L. Yan, Y. Zhao, Y. Wang, W. Zhang, J. Fan, X. Chen, Graphitic carbon nitride solid nanofilms for selective and recyclable sensing of Cu2+ and Ag+ in water and serum. Chem. Commun. 50(97), 15415–15418 (2014). doi:10.1039/c4cc06659f
J. Tian, Q. Liu, A.M. Asiri, X. Sun, Y. He, Ultrathin graphitic C3N4 nanofibers: hydrolysis-driven top-down rapid synthesis and application as a novel fluorosensor for rapid, sensitive, and selective detection of Fe3+. Sens. Actuators B 216, 453–460 (2015). doi:10.1016/j.snb.2015.04.075
G. Shiravand, A. Badiei, G.M. Ziarani, Carboxyl-rich g-C3N4 nanoparticles: Synthesis, characterization and their application for selective fluorescence sensing of Hg2+ and Fe3+ in aqueous media. Sens. Actuators B 242, 244–252 (2017). doi:10.1016/j.snb.2016.11.038
S. Barman, M. Sadhukhan, Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J. Mater. Chem. 22(41), 21832–21837 (2012). doi:10.1039/C2JM35501A
X.L. Zhang, C. Zheng, S.S. Guo, J. Li, H.H. Yang, G. Chen, Turn-on fluorescence sensor for intracellular imaging of glutathione using g-C(3)N(4) nanosheet-MnO(2) sandwich nanocomposite. Anal. Chem. 86(7), 3426–3434 (2014). doi:10.1021/ac500336f
Y. Xu, X. Niu, H. Zhang, L. Xu, S. Zhao, H. Chen, X. Chen, Switch-on fluorescence sensing of glutathione in food samples based on a graphitic carbon nitride quantum dot (g-CNQD)–Hg2+ chemosensor. J. Agric. Food Chem. 63(6), 1747–1755 (2015). doi:10.1021/jf505759z
J. Han, H.Y. Zou, M.X. Gao, C.Z. Huang, A graphitic carbon nitride based fluorescence resonance energy transfer detection of riboflavin. Talanta 148, 279–284 (2016). doi:10.1016/j.talanta.2015.10.038
Q.-M. Feng, Y.-Z. Shen, M.-X. Li, Z.-L. Zhang, W. Zhao, J.-J. Xu, H.-Y. Chen, Dual-wavelength electrochemiluminescence ratiometry based on resonance energy transfer between Au nanoparticles functionalized g-C3N4 nanosheet and Ru (bpy) 32+ for microRNA detection. Anal. Chem. 88(1), 937–944 (2015). doi:10.1021/acs.analchem.5b03670
Y. Tang, H. Song, Y. Su, Y. Lv, Turn-on persistent luminescence probe based on graphitic carbon nitride for imaging detection of biothiols in biological fluids. Anal. Chem. 85(24), 11876–11884 (2013). doi:10.1021/ac403517u
D. Das, S.L. Shinde, K.K. Nanda, Temperature-dependent photoluminescence of g-C3N4: implication for temperature sensing. ACS Appl. Mater. Interfaces 8(3), 2181–2186 (2016). doi:10.1021/acsami.5b10770
L. Feng, F. He, G. Yang, S. Gai, Y. Dai, C. Li, P. Yang, NIR-driven graphitic-phase carbon nitride nanosheets for efficient bioimaging and photodynamic therapy. J. Mater. Chem. B 4(48), 8000–8008 (2016). doi:10.1039/c6tb02232d
L. Feng, F. He, B. Liu, G. Yang, S. Gai, P. Yang, C. Li, Y. Dai, R. Lv, J. Lin, g-C3N4 coated upconversion nanoparticles for 808 nm near-infrared light triggered phototherapy and multiple imaging. Chem. Mater. 28(21), 7935–7946 (2016). doi:10.1021/acs.chemmater.6b03598
A. Wang, C. Lee, H. Bian, Z. Li, Y. Zhan, J. He, Y. Wang, J. Lu, Y.Y. Li, Synthesis of g-C3N4/silica gels for white-light-emitting devices. Part. Part. Syst. Charact. 34(1), 1600258 (2017). doi:10.1002/ppsc.201600258
S. Bayan, N. Gogurla, A. Midya, S.K. Ray, White light emission characteristics of two dimensional graphitic carbon nitride and ZnO nanorod hybrid heterojunctions. Carbon 108, 335–342 (2016). doi:10.1016/j.carbon.2016.07.032
J. Tian, Q. Liu, A.M. Asiri, K.A. Alamry, X. Sun, Ultrathin graphitic C3N4 nanosheets/graphene composites: efficient organic electrocatalyst for oxygen evolution reaction. Chemsuschem 7(8), 2125–2130 (2014). doi:10.1002/cssc.201402118
J. Tian, R. Ning, Q. Liu, A.M. Asiri, A.O. Al-Youbi, X. Sun, Three-dimensional porous supramolecular architecture from ultrathin g-C(3)N(4) nanosheets and reduced graphene oxide: solution self-assembly construction and application as a highly efficient metal-free electrocatalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 6(2), 1011–1017 (2014). doi:10.1021/am404536w
J. Tian, Q. Liu, A.M. Asiri, A.H. Qusti, A.O. Al-Youbi, X. Sun, Ultrathin graphitic carbon nitride nanosheets: a novel peroxidase mimetic, Fe doping-mediated catalytic performance enhancement and application to rapid, highly sensitive optical detection of glucose. Nanoscale 5(23), 11604–11609 (2013). doi:10.1039/c3nr03693f
J. Tian, Q. Liu, C. Ge, Z. Xing, A.M. Asiri, A.O. Al-Youbi, X. Sun, Ultrathin graphitic carbonnitride nanosheets: a low-cost, green, and highly efficient electrocatalyst toward the reduction of hydrogen peroxide and its glucose biosensing application. Nanoscale 5(19), 8921–8924 (2013). doi:10.1039/C3NR02031B
L. Shi, L. Liang, F.X. Wang, J. Ma, J.M. Sun, Polycondensation of guanidine hydrochloride into a graphitic carbon nitride semiconductor with a large surface area as a visible light photocatalyst. Catal. Sci. Technol. 4(9), 3235–3243 (2014). doi:10.1039/C4CY00411F
B.H. Long, J.L. Lin, X.C. Wang, Thermally-induced desulfurization and conversion of guanidine thiocyanate into graphitic carbon nitride catalysts for hydrogen photosynthesis. J. Mater. Chem. A 2(9), 2942–2951 (2014). doi:10.1039/c3ta14339b
S. Yuan, Q. Zhang, B. Xu, S. Liu, J. Wang, J. Xie, M. Zhang, T. Ohno, A new precursor to synthesize g-C3N4 with superior visible light absorption for photocatalytic application. Catal. Sci. Technol. 7(9), 1826–1830 (2017). doi:10.1039/c7cy00213k
J.S. Zhang, F.S. Guo, X.C. Wang, An optimized and general synthetic strategy for fabrication of polymeric carbon nitride nanoarchitectures. Adv. Funct. Mater. 23(23), 3008–3014 (2013). doi:10.1002/adfm.201203287
L. Shi, L. Liang, F.X. Wang, M.S. Liu, K.L. Chen, K.N. Sun, N.Q. Zhang, J.M. Sun, Higher yield urea-derived polymeric graphitic carbon nitride with mesoporous structure and superior visible-light-responsive activity. ACS Sustain. Chem. Eng. 3(12), 3412–3419 (2015). doi:10.1021/acssuschemeng.5b01139
J.H. Sun, J.S. Zhang, M.W. Zhang, M. Antonietti, X.Z. Fu, X.C. Wang, Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles. Nat. Commun. 3, 1139 (2012). doi:10.1038/Ncomms2152
H. Yan, Soft-templating synthesis of mesoporous graphitic carbon nitride with enhanced photocatalytic H2 evolution under visible light. Chem. Commun. 48(28), 3430–3432 (2012). doi:10.1039/c2cc00001f
Q.J. Fan, J.J. Liu, Y.C. Yu, S.L. Zuo, A template induced method to synthesize nanoporous graphitic carbon nitride with enhanced photocatalytic activity under visible light. RSC Adv. 4(106), 61877–61883 (2014). doi:10.1039/C4RA12033G
L. Shi, L. Liang, F. Wang, M. Liu, T. Liang, K. Chen, J. Sun, In situ bubble template promoted facile preparation of porous g-C3N4 with excellent visible-light photocatalytic performance. RSC Adv. 5(78), 63264–63270 (2015). doi:10.1039/C5RA09645F
F. He, G. Chen, Y. Yu, Y. Zhou, Y. Zheng, S. Hao, The sulfur-bubble template-mediated synthesis of uniform porous g-C3N4 with superior photocatalytic performance. Chem. Commun. 51(2), 425–427 (2015). doi:10.1039/C4CC07106A
J. Xu, Y. Wang, Y. Zhu, Nanoporous graphitic carbon nitride with enhanced photocatalytic performance. Langmuir 29(33), 10566–10572 (2013). doi:10.1021/la402268u
M. Zhang, J. Xu, R. Zong, Y. Zhu, Enhancement of visible light photocatalytic activities via porous structure of g-C3N4. Appl. Catal. B 147, 229–235 (2014). doi:10.1016/j.apcatb.2013.09.002
H. Xu, J. Yan, Y. Xu, Y. Song, H. Li, J. Xia, C. Huang, H. Wan, Novel visible-light-driven AgX/graphite-like C3N4 (X = Br, I) hybrid materials with synergistic photocatalytic activity. Appl. Catal. B 129, 182–193 (2013). doi:10.1016/j.apcatb.2012.08.015
Y. Li, L. Fang, R. Jin, Y. Yang, X. Fang, Y. Xing, S. Song, Preparation and enhanced visible light photocatalytic activity of novel g-C3N4 nanosheets loaded with Ag2CO3 nanoparticles. Nanoscale 7(2), 758–764 (2015). doi:10.1039/c4nr06565d
S. Zhan, F. Zhou, N. Huang, Y. Yang, Y. Liu, Y. Yin, Y. Fang, g-C3N4/ZnWO4 films: preparation and its enhanced photocatalytic decomposition of phenol in UV. Appl. Surf. Sci. 358, 328–335 (2015). doi:10.1016/j.apsusc.2015.07.180
L. Shi, L. Liang, J. Ma, F. Wang, J. Sun, Enhanced photocatalytic activity over the Ag2O–g-C3N4 composite under visible light. Catal. Sci. Technol. 4(3), 758–765 (2014). doi:10.1039/c3cy00871a
M.J. Muñoz-Batista, O. Fontelles-Carceller, M. Ferrer, M. Fernández-García, A. Kubacka, Disinfection capability of Ag/g-C3N4 composite photocatalysts under UV and visible light illumination. Appl. Catal. B 183, 86–95 (2016). doi:10.1016/j.apcatb.2015.10.024
L. Shi, L. Liang, J. Ma, F. Wang, J. Sun, Remarkably enhanced photocatalytic activity of ordered mesoporous carbon/gC3N4 composite photocatalysts under visible light. Dalton Trans. 43(19), 7236–7244 (2014). doi:10.1039/C4DT00087K
P. Gibot, F. Schnell, D. Spitzer, Enhancement of the graphitic carbon nitride surface properties from calcium salts as templates. Micropor. Mesopor. Mater. 219, 42–47 (2016). doi:10.1016/j.micromeso.2015.07.026
X. Gao, X. Jiao, L. Zhang, W. Zhu, X. Xu, H. Ma, T. Chen, Cosolvent-free nanocasting synthesis of ordered mesoporous g-C3N4 and its remarkable photocatalytic activity for methyl orange degradation. RSC Adv. 5(94), 76963–76972 (2015). doi:10.1039/C5RA13438B
J. Wen, J. Xie, X. Chen, X. Li, A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 391, 72–123 (2017). doi:10.1016/j.apsusc.2016.07.030
K. Vignesh, S. Kang, B.S. Kwak, M. Kang, Meso-porous ZnO nano-triangles@ graphitic-C3N4 nano-foils: fabrication and Recyclable photocatalytic activity. Sep. Purif. Technol. 147, 257–265 (2015). doi:10.1016/j.seppur.2015.04.043
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, A., Wang, C., Fu, L. et al. Recent Advances of Graphitic Carbon Nitride-Based Structures and Applications in Catalyst, Sensing, Imaging, and LEDs. Nano-Micro Lett. 9, 47 (2017). https://doi.org/10.1007/s40820-017-0148-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-017-0148-2