Abstract
Introduction
Both mycophenolate mofetil (MMF) and intravenous cyclophosphamide (CYC) have been recommended in the induction therapy of lupus nephritis (LN) for years; nevertheless, their effectiveness and safety in a real-world setting are extremely lacking. Therefore, we decided to conduct this real-world study.
Methods
A total of 195 Chinese patients with LN who were initially treated with MMF (n = 98), or intravenous CYC (n = 97) as induction therapy were enrolled. All of the patients were followed up to 12 months. Complete renal remission (CRR) was defined as 24-h urinary protein (24 h-UTP) < 0.5 g, and partial renal remission (PRR) was defined as ≥ 50% reduction in 24 h-UTP to the subnephrotic level, however > 0.5 g, both with a change of serum creatinine (SCr) within 10% from baseline. The proportions of CRR, PRR, and total renal remission (TRR), as well as adverse events, were compared by Chi-square test and Kaplan–Meier analysis (log-rank test). Inverse probability of treatment weighting (IPTW) was used for propensity score matching and multivariable logistic regression analyses were employed.
Results
The cumulative proportion of TRR in 6 months (79.4 vs. 63.8%, p = 0.026) and CRR in 12 months (72.8 vs. 57.6%, p = 0.049) in MMF group were significantly higher than CYC group, and the above conclusions were further confirmed by IPTW. The proportions of PRR, CRR, and TRR at other time points were equivalent between two groups. Further subgroup analysis in 111 patients with biopsy-proven III–V LN also showed a significantly higher proportion of TRR at 6 months in the MMF group than in the CYC group (78.3 vs. 56.9%, p = 0.026). In the Kaplan–Meier analysis and after IPTW, the MMF group showed better TRR and CRR responses than CYC group in 12 months. Multivariable logistic regression analyses revealed that MMF use was the only predictor of CRR (HR 2.12, 95% CI 1.90–4.09, p = 0.026), while low complement level was also a predictor, albeit risk was reduced (HR 0.38, 95% CI 0.17–0.86, p = 0.019). Moreover, compared to the CYC group, MMF group patients were more likely to have significantly lower SCr (μmol/l) [72.5 (62.5, 86.5) vs. 79.0 (71.1, 97.5), p = 0.001] and daily dose of prednisone (mg/day) (15.7 ± 5.2 vs. 18.6 ± 11.3, p = 0.022) at 6 months; lower 24 h-UTP (g) [0.1 (0.1, 0.3) vs. 0.2 (0.1, 0.9), p = 0.005] and daily dose of prednisone (mg/day) (9.6 ± 3.3 vs. 11.2 ± 5.5, p = 0.023) at 12 months. Infection was the most common adverse event. Pneumonia and gastrointestinal discomfort were more frequently observed in the CYC group.
Conclusions
Real-world data are a key component of the evidence supporting the effectiveness of drugs and are of interest to all stakeholders. Our comparative study demonstrated the effectiveness of MMF in LN induction therapy was at least equivalent to intravenous CYC, with superior tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although both mycophenolate mofetil (MMF) and intravenous cyclophosphamide (CYC) have been widely used in patients with lupus nephritis for years based on their similar efficacy shown in randomized controlled trials, there is little real-world data so far. |
What was learned from this study? |
Our study suggested the effectiveness of MMF was at least equivalent to intravenous CYC as an induction therapy for lupus nephritis, even at relatively low doses. |
MMF was associated with better tolerance compared to CYC, with less pneumonia, gastrointestinal adverse reactions, and menstrual disturbance. |
Introduction
Renal disease in systemic lupus erythematosus (SLE) carries significant morbidity and mortality [1,2,3]. Mycophenolate mofetil (MMF) and cyclophosphamide (CYC) have been recommended in the induction treatment for lupus nephritis (LN) by several guidelines, based on their similar efficacy shown in randomized controlled trials [4,5,6]. However, some studies even showed the superiority of MMF to CYC [7, 8]. A relatively low dose of MMF has been widely used in Chinese patients, and the clinical effect needs the corresponding data to support. Furthermore, some clinicians believe that Chinese patients not only respond well but also show better tolerance to CYC compared to MMF due to racial disparity [3, 9].
Despite the widespread use of both MMF and CYC in clinical practice for several years, there is currently limited real-world data available. Therefore, we conducted a real-world study to compare the effectiveness and safety between MMF and CYC in the induction therapy in patients with LN.
Methods
Study Population
All the patients in this study were enrolled from the Peking University First Hospital SLE (PKUFHS) cohort, a longitudinal observational cohort set up in 2007. They were at least 18 years old with fulfilment of the 1999 American College of Rheumatology or 2012 systemic lupus international collaborating clinics classification criteria for SLE. All patients were regularly followed up every 1–3 months when the disease was active or every 3–6 months when lupus was stable. More details of the cohort have been previously described [10]. In this study, we enrolled the patients who met the 2003 ISN/RPS classification criteria of LN, and received oral MMF alone (MMF group) or intravenous CYC alone (CYC group) in addition to glucocorticoids (GC) as initial induction therapy for LN from the PKUFHS cohort. The time point when a patient with active LN initiated a high dose of glucocorticoid combined MMF or intravenous CYC as induction therapy was defined as the baseline visit in the study.
The study was approved by the ethical committee of Peking University First Hospital [PKUFH-2017(1284)] and written informed consent was obtained from each patient at enrolment. All procedures performed in studies involving human participants in this study were performed in accordance with the Helsinki Declaration of 1964 and its later amendments or comparable ethical standards.
Data Collection
The demographics, clinical and laboratory characteristics as well as treatments were prospectively collected. Lab results included complete blood counts, chemistry, especially serum creatinine (SCr), serum albumin (Alb), complements (C3 and C4), autoantibodies, especially antinuclear antibody (ANA), anti-double-strand DNA (anti-dsDNA), anti-extractable nuclear antigen (anti-ENA), anti-phospholipid antibodies (aPL), 24-h urinary protein (24 h-UTP), and urine sediments. The medications, including GC, MMF or CYC, hydroxychloroquine (HCQ), angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB), were recorded. At each visit, SLE disease activity index 2000 (SLEDAI-2K) was assessed, the dosage of prescribed GC was checked, and converted to prednisone equivalent dose. Safety evaluation included the assessment of vital signs, lab results, and spontaneous reports of adverse events (AEs).
Study Outcomes
We analyzed the achievement of complete renal remission (CRR) and partial renal remission (PRR) at 12 months after initiation of induction therapy. CRR was defined as 24 h-UTP < 0.5 g with a change of SCr within 10% from baseline. PRR was defined as ≥ 50% reduction in 24 h-UTP to the subnephrotic level, however > 0.5 g with a change of SCr within 10% from baseline [4]. Total renal remission (TRR) was the sum of CRR and PRR. The proportions of patients achieving CRR, PRR, or TRR in 6 and 12 months were the primary outcomes of the study. Comparisons of other indicators between two groups were also conducted, including 24 h-UTP, SCr, Alb, anti-dsDNA positivity, serum levels of C3, SLEDAI, GC daily dose, lupus low disease activity status (LLDAS) achievement, as well as AE [11]. Subgroup analysis was done in patients with biopsy-proven III-V LN.
Statistical Analysis
Categorical variables were described as numbers of cases (percentage). Normally distributed continuous variables were presented as the means ± standard deviations, and continuous variables deviating from the normal distribution were presented as the median with quartile values. Comparisons of continuous variables were made using Student’s t test or Mann–Whitney U test where appropriate. Categorical variables were compared by Chi-square test or Fisher’s exact probability test. Rates of remission was compared by Kaplan–Meier survival curves (log-rank test). Risk factors for CRR were conducted by logistic regression analysis. All factors were examined using univariable analysis, and factors that were statistically significant on the univariable analysis were analyzed by multivariable analysis. The results were analyzed on the basis of intention-to-treat. All testing analysis was bilateral, and p < 0.05 was considered statistically significant. Data analyses were performed with SPSS 26.0 (SPSS IBM Corp., Armonk, NY, USA) and RStudio software (version 4.2.1).
Inverse probability of treatment weighting (IPTW) based on the propensity score was adopted to adjust for the baseline characteristics between the two groups. The calculated propensity scores were weighted using the “ratio of patients receiving CYC to all patients/propensity score” in the CYC group and the “ratio of patients receiving MMF to all patients/1-propensity score” in the MMF group as the weighting coefficient on stability. To calculate the propensity scores, multivariable logistic regression analysis was performed with the concomitant use of CYC as the dependent variable and the following as independent variables: age of SLE onset, SLE duration, 24 h-UTP, Scr, HCQ usage, and ACEI/ARB usage.
Results
Demographics and Clinical Features of Enrolled Patients at Baseline
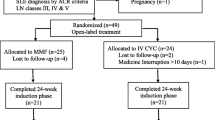
A total of 360 Chinese patients with LN from July 1, 2010 to June 30, 2019 were screened, and 195 patients fulfilling the inclusion criteria were finally included for analysis (Fig. 1).
The average age of 195 patients was 35.0 years. The median SLE disease duration was 2.9 (0.3, 7.1) years with SLEDAI 17.8 ± 5.9. There were 163 (83.6%) females and 100 (51.3%) treatment-naïve patients. Renal involvement (133, 68.6%) was the most common initial presentation, followed by hematological abnormalities (104, 53.3%), rash (104, 53.3%), fever (101, 52.1%), arthritis (95, 49.0%), alopecia (73, 37.6%), serositis (37, 19.1%), myositis (32, 16.5%), oral ulceration (28, 14.4%), and neuropsychiatric manifestations (5, 2.6%). ANA was positive in all patients, and anti-dsDNA was positive in 190 (97.9%) patients. Antibodies against SSA, SSB, Sm, nRNP, and rRNP were respectively detectable in 113 (57.9%), 36 (18.5%), 64 (32.8%), 80 (41.0%), and 54 (27.7%) patients. Regarding anti-phospholipid antibodies, anti-β2-glycoprotein I, lupus anticoagulant, and anticardiolipin antibody were presented in 37 (28.5%), 32 (23.4%), and 19 (12.3%) patients.
There were 98 patients in the MMF group and 97 patients in the CYC group. As for the patients with new-onset LN, there was no statistical difference between MMF and CYC groups [46 (46.9%) vs. 54 (55.7%); p = 0.223]. For induction therapy, MMF was administered at dosages of 2 g/day in 14 (14.3%) patients, 1.5 g/day in 75 (76.5%) patients, and 1.0 g/day in 9 (9.2%) patients. MMF was usually escalated to the target dose in 2–4 weeks. As for CYC, intravenous 0.4 g biweekly was used in 67 (69.1%) patients, 0.8–1.0 g monthly in 16 (16.5%) patients, and other regimens in 14 (14.4%) patients. There was no significant difference in initial presentations and laboratory profiles between the two groups. However, patients in the MMF group tended to be younger with a shorter disease duration.
The initial dose of prednisone in 195 patients was 60.1 ± 20.2 mg/day. Totally, 150 (76.9%) patients received HCQ, and 134 (68.7%) patients received ACEI/ARB treatment. There were more patients in the MMF group who received HCQ and ACEIs/ARBs compared to CYC group (Table 1).
The Improvement in Disease Activity in All Patients Throughout 12 Months
Significant improvements in disease activity relevant parameters, including SLEDAI, 24 h-UTP, urine sediments, SCr, Alb, ratio of anti-dsDNA positivity, serum levels of C3, were observed at 6 and 12 months from baseline in both groups. For instance, a major decrease in SLEDAI was observed, from 17.8 ± 5.9 at enrollment to 6.8 ± 4.2 at 6 months and 5.1 ± 4.5 at 12 months in 195 patients. The median 24 h-UTP was decreased from 3.4 (1.8, 4.5) g at baseline to 0.4 (0.1, 1.4) g at 6 months and 0.2 (0.1, 0.6) g at 12 months, with the corresponding elevation of serum albumin from 28.2 ± 6.5 g/l to 38.3 ± 5.4 g/l at 6 months and 40.6 ± 4.2 g/l at 12 months. The SCr remained stable throughout the entire 12 months of follow-up.
Meanwhile, the daily dose of prednisolone was decreased from 60.1 ± 20.2 mg to 17.2 ± 8.9 mg at 6 months and 10.4 ± 4.6 mg/day at 12 months.
Comparisons of Renal Remission Rates Between MMF and CYC Groups
Both 6-month cumulative CRR and PRR in MMF group were not significantly different from those in CYC group [CRR: 46 (47.4%) vs. 35 (37.2%), p = 0.224; PRR: 31 (32.0%) vs. 25 (26.6%), p = 0.512], but the cumulative TRR in MMF group was significantly higher compared to CYC group [77 (79.4%) vs. 60 (63.8%), p = 0.026].
In 12 months, the cumulative proportion of CRR was significantly higher in MMF group compared to CYC group [67 (72.8%) vs. 49 (57.6%), p = 0.049], however, the corresponding proportions of PRR and TRR in MMF group were comparable to those in CYC group [PRR: 15 (16.3%) vs. 22 (25.0%), p = 0.208; TRR: 82 (89.1%) vs. 71 (83.5%), p = 0.245].
Similar results were obtained after control for baseline characteristics by IPTW based on the propensity score (Table 2).
The Kaplan–Meier curves depicting the cumulative proportions of renal remission are shown in Fig. 2. Most of the cumulative CRR, PRR, and TRR in 6 months and 12 months in MMF group were not different from those in CYC group [6 months, CRR: 46 (46.9%) vs. 36 (37.5%), p = 0.225; PRR: 32 (32.7%) vs. 25 (26.0%), p = 0.328; 12 months, CRR: 67 (70.5%) vs. 51 (55.6%), p = 0.056; PRR: 16 (16.7%) vs. 22 (23.3%), p = 0.237; TRR: 83 (85.6%) vs. 73 (77.0%), p = 0.066], except that the cumulative TRR in MMF group was significantly higher compared to CYC group in 6 months [78 (80.4%) vs. 61 (63.3%), p = 0.016].
Comparisons of Other Measures Between Two Groups
Compared to the CYC group, the patients in MMF group had significantly lower daily dose of prednisone (mg/day) at 6 and 12 months (15.7 ± 5.2 vs. 18.6 ± 11.3, p = 0.022; 9.6 ± 3.3 vs. 11.2 ± 5.5, p = 0.023, respectively), and the trend remained after IPTW (Supplementary Material: Tables S1 and S2). There was no significant difference in SLEDAI between the two groups (6 months: 6.8 ± 3.8 vs. 6.8 ± 4.5, p = 0.910; 12 months: 4.8 ± 4.1 vs. 5.3 ± 4.9, p = 0.441).
As for lab parameters, patients in MMF group had significantly higher counts of white blood cells (× 109/l) (12 months: 6.7 ± 2.9 vs. 5.5 ± 2.1, p = 0.004), higher platelet counts (× 109/l) (6 months: 243 ± 72.3 vs. 216.6 ± 67.2, p = 0.010; 12 months: 239.1 ± 57.1 vs. 215.5 ± 61.1, p = 0.012), lower 24-hUTP (g) [12 months: 0.1 (0.1, 0.3) vs. 0.2 (0.1, 0.9), p = 0.005] and SCr (μmol/l) [6 months: 72.5 (62.5, 86.5) vs. 79.0 (71.1, 97.5), p = 0.001].
Two patients in each group achieved LLDAS in 6 months. Twelve (13.3%) patients in MMF group and nine (10.3%) patients in CYC group achieved LLDAS in 12 months.
Subgroup Analysis in Patients with Biopsy-Proven Class III-V Lupus Nephritis
Renal biopsy was performed in 112 (57.4%) patients. Their pathological patterns were classified as class II (one patient), III (13 patients), IV (40 patients), V (20 patients), III + V (15 patients), and IV + V (23 patients). The distribution of pathologic patterns was similar in the MMF and CYC groups (Supplementary Material: Table S3).
Compared to the CYC group, the proportion of TRR in 6 months was significantly higher in MMF group before and after IPTW, and the TRR response in 12 months was also better in MMF group after IPTW (Supplementary Material: Table S4).
In the Kaplan–Meier analysis, the MMF group generally showed better TRR and CRR responses than CYC group [TRR in 6 months: 47 (79.7%) vs. 29 (56.9%), p = 0.013; TRR in 12 months: 50 (84.2%) vs. 35 (70.5%), p = 0.039; CRR in 12 months: 40 (69.7%) vs. 25 (52.6%), p = 0.047] (Fig. 3).
In comparison with the CYC group, the patients in the MMF group had lower SCr (μmol/l) [6 months: 75.0 (62.0, 87.0) vs. 78.0 (71.1, 96.0), p = 0.026], lower 24-h UTP (g) [6 months: 0.4 (0.1, 1.2) vs. 0.7 (0.4, 1.9), p = 0.041; 12 months: 0.1 (0.1, 0.3) vs. 0.3 (0.1, 0.9), p = 0.009], higher white blood cells (× 109/l) (12 months: 6.7 ± 3.2 vs. 5.5 ± 2.3, p = 0.004), and lower prednisolone daily dose (mg/day) (12 months: 9.4 ± 2.5 vs. 12.2 ± 6.9, p = 0.030) (Supplementary Material: Table S5-S6).
Risk Factors for CRR by Logistic Regression
Further analysis was performed to elucidate the risk factors for CRR. Univariable analysis showed that higher serum albumin, mucosal ulcers, and MMF treatment were associated with CRR achievement, while male sex, ACEI/ARB usage, hypocomplementemia, and higher 24 h-UTP at baseline were associated with reduced risk of CRR. The multivariable analysis indicated that MMF treatment was the only predictor of CRR (HR 2.16, 95% CI 1.90, 4.09, p = 0.026), and hypocomplementemia was related to low risk of CRR achievement (HR 0.38, 95% CI 0.17, 0.86, p = 0.019) (Table 3).
Safety Analysis
During 12-month follow-up, 97 (49.5%) patients experienced at least one AE. Infection was most common (34.9%), especially common cold or upper respiratory tract infections. Compared to the MMF group, pneumonia was more often observed in the CYC group (11.3 vs. 3.1%, p = 0.050). Moreover, gastrointestinal adverse reactions were often reported in the CYC group (16.3 vs. 2.1%, p < 0.001) and more patients experienced menstrual disturbance [11 (11.3%) vs. 4 (4.1%), p = 0.102] (Table 4).
Discussion
Renal remission following induction therapy has been confirmed to be associated with better long-term renal survival [12, 13]. Many observational studies and randomized trials have shown that MMF is as effective as low-dose intravenous CYC, however with less side effects [7, 14,15,16,17].
The first randomized controlled study exploring the effectiveness of MMF compared with CYC in proliferative LN was reported in 2000. Researchers found that the MMF regimen induced CRR in 81% of patients and PRR in 14% patients within 12 months, while the corresponding proportions in CYC followed by azathioprine group were 76 and 14%, respectively. This established the important role MMF in LN induction remission [18]. Several subsequent studies further confirmed the conclusion [17, 19,20,21]. For instance, in an international randomized, controlled trial involving 370 patients with LN, the primary efficacy endpoint at 24 weeks was achieved in 56.2% patients receiving MMF and 53.0% patients receiving intravenous CYC [21]. Some studies even showed the superiority of MMF to CYC in LN remission induction therapy [7, 8]. A recent meta-analysis on controlled trials involving 1989 patients with LN showed that the efficacy of MMF was superior to CYC in terms of CRR (RR = 1.231, 95% CI 1.055–1.437) and increasing level of C3 (SMD = 0.475, 95% CI 0.230–0.719) [22].
To date, there is only one real-world study published in 2021. In this Japanese study, the continuation rate, AEs, and reasons for discontinuation of MMF were investigated in 119 patients with SLE. Although 70 patients used MMF for LN in the cohort, the renal remission rate was not provided [23].
We for the first time compared the effectiveness and safety of MMF and CYC in a real-world setting. We found that the renal remission proportions in patients with LN who received MMF or CYC was similar to previous reports overall. The proportions of TRR in 6 months and CRR in 12 months in MMF group were dramatically higher compared to the CYC group. Importantly, the above results were further confirmed by IPTW.
In patients with proliferative LN, we also found that TRR was achieved in significantly more patients in the MMF group compared to the CYC group in 6 months. Moreover, the patients in the MMF group showed better cumulative TRR and CRR responses than the CYC group in 12 months by IPTW. In addition, patients in the MMF group had significantly lower 24-hUTP, higher counts of white blood cells and platelets, lower SCr, and daily dose of prednisone compared to the CYC group.
These results have confirmed the effectiveness of MMF in the induction therapy for patients with active LN. To be noted is that a relatively low dose of MMF was adopted, with 1.5 g/day in majority of our patients, in contrast to 3 g/day in most previous studies in Caucasian and some Asian patients [7, 18, 21, 24, 25]. This reflects the real-world application of MMF in China. Nevertheless, our real-life data showed that the effectiveness of MMF was seemingly superior to CYC in inducing active LN into remission, which was in line with Sedhain’s findings [26].
ACEIs and ARBs have been widely administered to patients with diabetic and nondiabetic nephropathy because of their roles in renal protection and proteinuria reduction [27, 28]. In this real-world study including 135 patients who used ACEIs/ARBs and 60 patients who did not use, we surprisingly found ACEIs/ARBs usage was unfavorable for LN remission in the univariable analysis. ACEIs/ARBs users had higher proteinuria level at baseline was the possible reason to explain the results. HCQ is one of the cornerstones of SLE treatment [4, 29]. A total of 149 (76.4%) patients were administered HCQ therapy in our study. We found that HCQ usage was associated with decreased SCr level and increased opportunity to achieve cumulative PRR in 6 months, but we were not able to confirm the benefit of HCQ in other aspects, such as CRR achievement, SLEDAI, UTP, prednisone dose, and so on (data not shown).
Regarding safety, we observed infection as the most common AE, and patients in the CYC group were especially more susceptible to pneumonia. More AEs occurred in the CYC group, including gastrointestinal discomfort, nausea, vomiting, elevated aminotransferase, and menstrual disturbance. Hemorrhagic cystitis, though not rare in Caucasians, has never been reported in our patients [30].
This is the first real-word study to compare the effectiveness and safety of MMF and CYC in patients with LN. We found that the relatively low-dose MMF was at least as effective as CYC in induction therapy for LN. Among the patients with proliferative LN, we even observed higher renal total remission rate in MMF group. Multivariable logistic regression analyses confirmed that MMF application was the only predictor of CRR. Our research also has some limitations. Firstly, the inclusion of 195 patients in the study represents a relatively small sample size for a real-world study. However, it is worth noting that this is based on the largest number of patients with LN to date. Second, it was a single-center study in Chinese patients, which may limit the generalization of our findings to other populations. Third, we did not pre-protocolize the treatment regimens in this real-world study. HCQ and ACEI/ARB were more frequently used in the MMF group. The nonuniform treatment regimen might affect the effectiveness and safety. However, we used the IPTW approach to adjust the baseline characteristics between the two groups, which maximally reduced the potential bias. Fourth, some patients failed to complete the whole 12-month visit. Therefore, we chose Kaplan–Meier analysis to make full use of the data. Lastly, conducting multiple tests on the same dataset may increase the likelihood of obtaining at least one invalid result, which poses a significant risk of drawing incorrect conclusions.
Conclusions
Our real-life study indicates that induction therapy with MMF, even at a low dose, leads to at least equivalent effectiveness and better tolerance than intravenous CYC in patients with LN. MMF application is the only predictor of CRR of LN and low complement level was a predictor too, albeit risk was reduced.
References
Waldman M, Appel GB. Update on the treatment of lupus nephritis. Kidney Int. 2006;70(8):1403–12.
Font J, Ramos-Casals M, Cervera R, Garcia-Carrasco M, Torras A, Siso A, Darnell A, Ingelmo M. Cardiovascular risk factors and the long-term outcome of lupus nephritis. QJM. 2001;94(1):19–26.
Mok CC, Ying KY, Ng WL, Lee KW, To CH, Lau CS, Wong RW, Au TC. Long-term outcome of diffuse proliferative lupus glomerulonephritis treated with cyclophosphamide. Am J Med. 2006;119(4):355.e25-355.e333.
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, Cervera R, Doria A, Gordon C, Govoni M, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–45.
Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, Cook HT, Fervenza FC, Gibson KL, Glassock RJ, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):753–79.
Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64(6):797–808.
Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–28.
Elliott JR, Manzi S. Induction therapy for active lupus nephritis: mycophenolate mofetil is superior to cyclophosphamide. Nat Clin Pract Rheumatol. 2006;2(7):354–5.
Li J, Dai G, Zhang Z. General adverse response to cyclophosphamide in Chinese patients with systemic autoimmune diseases in recent decade-a single-center retrospective study. Clin Rheumatol. 2015;34(2):273–8.
Gao D, Hao Y, Mu L, Xie W, Fan Y, Ji L, Zhang Z. Frequencies and predictors of the Lupus Low Disease Activity State and remission in treatment-naive patients with systemic lupus erythematosus. Rheumatology (Oxford). 2020;59(11):3400–7.
Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, Wahono CS, Chen SL, Jin O, Morton S, et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann Rheum Dis. 2016;75(9):1615–21.
Medina-Rosas J, Fung WA, Su J, Touma Z. Effect of complete or partial proteinuria recovery on long-term outcomes of lupus nephritis. Semin Arthritis Rheum. 2018;47(4):557–64.
Korbet SM, Lewis EJ, Collaborative Study G. Severe lupus nephritis: the predictive value of a >/= 50% reduction in proteinuria at 6 months. Nephrol Dial Transplant. 2013;28(9):2313–8.
Rathi M, Goyal A, Jaryal A, Sharma A, Gupta PK, Ramachandran R, Kumar V, Kohli HS, Sakhuja V, Jha V, et al. Comparison of low-dose intravenous cyclophosphamide with oral mycophenolate mofetil in the treatment of lupus nephritis. Kidney Int. 2016;89(1):235–42.
Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G, Ghazalli R, Teo SM, Wong HS, Tan SY, Shaariah W, et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology (Carlton). 2005;10(5):504–10.
Wang J, Hu W, Xie H, Zhang H, Chen H, Zeng C, Liu Z, Li L. Induction therapies for class IV lupus nephritis with non-inflammatory necrotizing vasculopathy: mycophenolate mofetil or intravenous cyclophosphamide. Lupus. 2007;16(9):707–12.
Gadakchi L, Hajialilo M, Nakhjavani MR, Abedi Azar S, Kolahi S, Gojazadeh M, Ebrahimi AA, Malek Mahdavi A, Noshad H, Khabbazi A. Efficacy and safety of mycophenolate mofetil versus intravenous pulse cyclophosphamide as induction therapy in proliferative lupus nephritis. Iran J Kidney Dis. 2018;12(5):288–92.
Chan TM, Li FK, Tang CS, Wong RW, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000;343(16):1156–62.
El-Shafey EM, Abdou SH, Shareef MM. Is mycophenolate mofetil superior to pulse intravenous cyclophosphamide for induction therapy of proliferative lupus nephritis in Egyptian patients? Clin Exp Nephrol. 2010;14(3):214–21.
Mak A, Cheak AA, Tan JY, Su HC, Ho RC, Lau CS. Mycophenolate mofetil is as efficacious as, but safer than, cyclophosphamide in the treatment of proliferative lupus nephritis: a meta-analysis and meta-regression. Rheumatology (Oxford). 2009;48(8):944–52.
Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sanchez-Guerrero J, Solomons N, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–12.
Jiang YP, Zhao XX, Chen RR, Xu ZH, Wen CP, Yu J. Comparative efficacy and safety of mycophenolate mofetil and cyclophosphamide in the induction treatment of lupus nephritis: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(38): e22328.
Abe Y, Tada K, Yamaji K, Tamura N. Real-world experience of safety of mycophenolate mofetil in 119 Japanese patients with systemic lupus erythematosus: a retrospective single-center study. Biomed Res Int. 2021;2021:8630596.
Gul H, Mushtaq MS, Salim B, Samreen S, Nasim A, Khan M. A comparison of mycophenolate mofetil and cyclophosphamide as lupus nephritis induction therapy. J Ayub Med Coll Abbottabad. 2020;32(4):454–8.
Mendonca S, Gupta D, Ali S, Gupta P. Mycophenolate mofetil or cyclophosphamide in Indian patients with lupus nephritis: which is better? A single-center experience. Saudi J Kidney Dis Transpl. 2017;28(5):1069–77.
Sedhain A, Hada R, Agrawal RK, Bhattarai GR, Baral A. Low dose mycophenolate mofetil versus cyclophosphamide in the induction therapy of lupus nephritis in Nepalese population: a randomized control trial. BMC Nephrol. 2018;19(1):175.
van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ, Boersma E. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin–angiotensin–aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33(16):2088–97.
Coleman CI, Weeda ER, Kharat A, Bookhart B, Baker WL. Impact of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on renal and mortality outcomes in people with type 2 diabetes and proteinuria. Diabet Med. 2020;37(1):44–52.
Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–8.
Bennett AH. Cyclophosphamide and hemorrhagic cystitis. J Urol. 1974;111(5):603–6.
Acknowledgements
The authors would like to thank Dr. Xueying Li for her statistical advice. This study was based on the contributions of all colleagues in our department during the in-patient care and outpatient visits over the past decade.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Zhuoli Zhang conceived, designed the study, and critically revised the manuscript. Xiaohui Zhang collected the data, performed the statistical analysis, and drafted the manuscript. Hong Huang, Dai Gao, Juan Zhao, Lanlan Ji, Yong Fan and Yanjie Hao contributed to the data collection and statistical analysis. All the authors read and approved the final manuscript.
Prior Presentation
Oral presentation (OP0283), Copenhagen, Denmark, EULAR Congress, 3 Jun 2022. (Zhang X, Huang H, Gao D, et alOP0283 "Mycophenolate Mofetil vs. Cyclophosphamide For Treatment Of Lupus Nephritis: A Single-Center Cohort Real-World Analysis". Annals of the Rheumatic Diseases 2022;81:188.)
Disclosures
Xiaohui Zhang, Hong Huang, Dai Gao, Juan Zhao, Lanlan Ji, Yong Fan, Yanjie Hao, Zhuoli Zhang have nothing to disclose.
Compliance with Ethics Guidelines
The study was approved by the ethical committee of Peking University First Hospital [PKUFH-2017(1284)] and written informed consent was obtained from each patient at enrolment. All procedures performed in studies involving human participants in this study were performed in accordance with the Helsinki Declaration of 1964 and its later amendments or comparable ethical standards.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, X., Huang, H., Gao, D. et al. Comparison of the Effectiveness and Safety of Mycophenolate Mofetil and Cyclophosphamide in Lupus Nephritis: Evidence from a Real-World Study. Rheumatol Ther 10, 1199–1213 (2023). https://doi.org/10.1007/s40744-023-00572-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-023-00572-y