Abstract
Background
Mycophenolate mofetil (MMF) has long been used to manage lupus nephritis. Despite research on its long-term efficacy, it is still warranted to conduct further investigation regarding its indications, safety and outcome. This study was intended to evaluate our proposed protocol in maintenance therapy with MMF.
Twenty-four lupus nephritis patients were registered prior to their receiving 3–6 month induction therapy with monthly iv pulses of cyclophosphamide (CYC), followed by 24 month maintenance therapy using MMF and steroid. We defined end points as achievement of complete and partial remission, relapse, refractory to therapy as well as end stage renal disease (ESRD) and death. Friedman and repeated measurement tests were used to assess the effect of treatment on parameters over time.
Complete renal remission was achieved in 79.16% until the end of the last follow up with an average period of 12.45 ± 7.37 months since treatment commenced. Significant statistical differences were seen regarding proteinuria, hematuria, leukocyturia, plasma creatinine, C3, C4 before and after therapy (P < 0.05): plasma creatinine and proteinurea falling from 0.96 ± 0.65 to 0.75 ± 0.19 mg/dl (P < 0.14) and from 1.64 ± 1.12 to 0.27 ± 0.60 gr/24 h (P < 0.001). By the end of 24-month, 95.8% of patients had been in remission. Four episodes of relapse ended in remission followed by retreatment. No life-threatening side effects were observed in 66.6% of patients with fourteen cases of infection (58.3%). None of them developed ESRD.
Maintenance therapy with MMF was shown to yield favorable outcome with minimal complications, in treating lupus nephritis (IRCT2012071710313N1).
Similar content being viewed by others
Introduction
Renal involvement occurs in approximately 40% of patients with systemic lupus erythematosus. Nephritis is the first manifestation of lupus in 3–6% of patients and the severity of renal injury determines its prognosis. Generally, survival in lupus patients is roughly 92% at 10 years after diagnosis. Proliferative renal involvement is among the most severe manifestations of lupus and without proper treatment it can lead to significant morbidity and mortality (Cameron 1999; Mak et al. 2007; Korbet et al. 2007; Contreras et al. 2005; Bernatsky et al. 2006; Hahn et al. 2012).
A logical combination of drugs to achieve specific therapeutic goals is of paramount significance in treating lupus nephritis. The core of treatment is based on the application of drugs with maximum efficacy and minimum toxicity in order to control of nephritis to the point of allowing a good quality of life, and non progression of renal disease.
Effective therapy is designed to reduce mortality and prevent progress to end-stage renal disease. Immunosuppressive regimens of glucocorticoids combined with cytotoxic drugs, particularly cyclophosphamide, was effective and standard for the treatment of severe proliferative lupus nephritis (Hahn et al. 2012; Contreras et al. 2004; Ferrantelli et al. 2005; Flanc et al. 2004; Austin et al. 1986; Illei et al. 2001; Gourley et al. 1996; Steinberg and Steinberg 2005; Rezaie-Yazdi et al. 2005; Mok et al. 2001; Ioannidis et al. 2000; Grootscholten et al. 2007). However, cyclophosphamide has both instant and cumulative adverse effects, including marrow suppression, gonadal toxicity, hemorrhagic cystitis, and the increased risk of cancer as well as the possibility of no response or relapse in several patients (Houssiau et al. 2002, 2010a). Therefore, other therapeutic agents, such as azathioprine and mycophenolate mofetile with few toxic effects, come prior to other alternatives (Grootscholten et al. 2006; Arends et al. 2012; Sahin et al. 2008; Houssiau et al. 2010b; Dooley et al. 2011).
Nowadays mycophenolate mofetile has been considered an important alternate agent for refractory lupus nephritis with hopeful results and reasonable side effects (Chan et al. 2000, 2005; Lenz et al. 2005; Weng et al. 2010; Zhu et al. 2007; Ong et al. 2005; Karim et al. 2005). Mycophenolic acid, the active metabolite of mycophenolate mofetil , selectively suppresses the proliferation of T and B lymphocyte, the formation of antibodies, and the glycosylation of adhesion molecules by inhibiting purine nucleotide synthesis and depleting lymphocytes and monocytes of guanosine triphosphate (Eickenberg et al. 2012). Mycophenolate mofetile as maintenance therapy after short-term intravenous cyclophosphamide have been shown efficient and safe, reducing the long-term exposure to cyclophosphamide (Bernatsky et al. 2006; Flores-Suárez 2006; Borba et al. 2006; Tse et al. 2006). In comparison with cyclophosphamide the adverse effects of MMF have been revealed to be well- tolerated, with gastrointestinal upset being the most common, and no mutagenic effects (Laskari et al. 2010; Elyan and Ballou 2009).
The aim of our study was to evaluate the efficacy and safety of mycophenolate mofetil combined with prednisolone for maintenance treatment of lupus nephritis in a single center cohort of patients with proliferative lupus nephritis.
Patients & methods
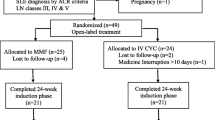
In this open label clinical trial twenty four consecutive patients with the diagnosis of lupus in accordance with ACR classification criteria (Tan et al. 1982) were enrolled and prospectively followed up during 24 months. All patients had the criteria for nephritis. Renal biopsy was done for 20 patients. Every specimen was observed under light microscopy, the findings of which were subsequently categorized based on the revised World Health Organization (WHO) classification for lupus nephritis (Weening et al. 2004).
Inclusion criteria were defined by one of the followings:
-
Evidence of active proliferative glomerulonephritis in the renal biopsy (WHO class IV, III)
-
In case of absent renal biopsy or the presence of WHO class V in biopsy, with the presence of the following clinical or paraclinical findings:
-
a)
Proteinaria >1 gr/24 h
-
b)
Progressive renal failure with 30% decrease in creatinine clearance over one-year period and creatinine >1.9 mg/dL
-
c)
The presence of more than 5 red cells in HPF of urine sediment in two separate specimens taken in a year, presence of WBC, granular or hyaline casts without active infection (was examined under conventional light microscope)
-
a)
Exclusion criteria include:
WHO class I or II lupus nephritis, end stage renal disease when replacement renal therapy will be indicated, leukopenia (neutrophils < 1500/mm3) due to bone marrow suppression, recurrent episodes of bacterial infection, history of cytotoxic drug treatment for more than two weeks or pulse therapy with corticosteroids during a six-week period before study entry.
Study protocol and immunosuppressive treatment
For classes III and IV of lupus nephritis, the treatment considered as four phases:
-
1)
Induction: intravenous cyclophosphamide, given as boluses once a month for 3–6 consecutive months (to the maximum dose of 1 g/m2) in addition to corticosteroid. All patients received ondansetron to prevent nausea and vomiting with every CYC pulse. High dose glucocorticoid was initiated with ivtravenous pulse methylprednisolon (500–1000 mg given over 30 minutes daily for three days) then changed to oral prednisolon (40–60 mg) for one month, tapered to intermediate dose during maintenance phase of therapy.
-
2)
Maintenance: mycophenolate mofetil (cellcept®, manufactured by Zahravi Pharm.Co, Under License of F. Hoffmann-La Roche Ltd, Basel, Switzerland) to the maximum dose of 2 gr/day combined with corticosteroid.
-
3)
Tapering: mycophenolate mofetil dosage remains unchanged for the first year followed by dose reduction in the second year, in the absence of relapse or partial remission fulfillment.
When partial remission was achieved, we started to taper prednisolon by an average of 10% monthly in order to reach an optimum dose of 5–10 mg/day.
-
4)
Discontinuation: if our study goals had been achieved, the mycophenolate mofetil would have been discontinued with the further tapering of prednisolone to the lowest possible daily dose and patients’ follow-up for evidence of relapse.
Those with class V lupus nephritis were given mycophenolate mofetil combined with corticosteroid since the diagnosis was made.
Patients were followed up every month during induction therapy and every other month during the first year and every three months thereafter. During each visit, the patients were evaluated including a complete physical examination as well as all laboratory and serologic tests (blood count, urine analysis, creatinine, GFR, measurement of proteinuria in 24 h urine collection, C3, C4, Anti-DNA, ANA, ESR). At each visit, side effects were investigated via enquiry and thorough physical examination. Each patient had a complete clinical evaluation for any other organ involvement besides nephritis as well as episodes of relapse.
Informed consent was obtained from all patients. The design of the work has been approved by the ethical committee of Mashhad University of Medical sciences.
End points were defined as
Criteria for complete remission:
-
a)
Complete improvement of renal and extra-renal symptoms:
Rise of creatinine less than 0.3 mg/dL, less than 300 mg proteinuria per day (only trace proteinuria) or less than 50% of initial proteinuria when Cr >1.7 mg/dl, absence of RBC cast, complete regression of all systemic symptoms
-
b)
Return to within normal limits of ESR, C3, C4, Hb and fall of autoantibody titers
-
c)
Absence of relapses and infectious complications
Criteria for partial remission:
-
a)
No progression of renal disease (normal or stable renal function)
-
b)
Decrease of at least 50% in dysmorphic RBC, cellular casts and proteinuria in the absence of doubling of serum creatinine or less than 1 gr proteinuria per day
-
c)
Return to within normal limits of markers of inflammation such as ESR
-
d)
Regression of systemic symptoms
-
e)
Return of patient to functional class 2 in the presence of an acceptable rate of complications
Relapse:
-
a)
More than 50% increase in (after reaching the lowest level during therapy) serum creatinine, dysmorphic RBC, cellular cast
-
b)
Doubling of proteinuria if there was nephritic proteinuria or at least 2 gr/day if based proteinuria < 3.5 gr/day
-
c)
At least two systemic symptoms reappeared
Refractory to therapy:
-
a)
No renal response in spite of 6 month treatment with mycophenolate mofetil
End stage renal disease:
-
a)
Plasma creatinine rose and got stabilized above 5 mg/dl for 3 months
Statistical analysis
Analyses of all data were conducted in SPSS version 11.5. The significance of the process of changes of variables with abnormal distribution was assessed by Friedman test whereas those with normal distribution were tested by repeated measurement.
Wilcoxon signed ranks test was used for comparing quantitative variables with abnormal distribution while Paired t-test was applied to variables with normal distribution. All mean values were shown ±1SD and P values < 0.05 were considered statistically significant.
Results
Patients’ characteristics
We enrolled 20 females and 4 males with active lupus nephritis with majority documented with diffuse proliferative glomerulonephritis. Two tissue samples (10%) were compatible with WHO class III, 13 samples were WHO class IV (65%) and 5 samples were WHO class V (25%). Adverse predictive factors such as proteinuria, low GFR, hypertention, were detected in 95.8%, 33.3% and 41.6% of patients respectively. At the beginning of renal disease 21 patients had hematuria and 18 patients had leukocyturia. The average range of proteinuria before treatment was 1.57 ± 1 gr/day. Three patients (13%) had nephrotic syndrome (proteinuria >3gr/day). Decrease in C3 and C4 levels were seen in 33.30% at the beginning of therapy. Anti DNA was positive in 50% of patients. Renal insufficiency means creatinine >1.9 mg/dL was seen in one patient at the beginning of therapy.
Extra renal manifestations at baseline are as follows: skin rash 13(54.2%), arthritis 15(62.5%), oral ulcer 16(66.7%), vasculitis 3(12.5%), nervous system involvement 3(13%), cardiac involvement 1(4.2%) and pneumonitis 1(4.2%). Baseline characteristics of 24 patients with lupus nephritis are shown in Table 1.
Results of the statistical comparisons among selected variables at baseline and at the latest follow up showed a significant improvement of all parameters including proteinuria, C3 and C4 (Table 2). Then we evaluated laboratory data variables changes during the course of treatment.
Significant alterations could be observed in proteinuria which resolved in 20 out of 24 (83.3%) patients (Figure 1), and hematuria (P < 0.001), whereas parameters such as platlete count, Hb and ESR seemed immune to drastic changes (P = 0.31, P = 0.88, P = 0.24). The creatinine level changes during the course of therapy has been shown in Figure 2 (P = 0.68). As to renal involvement implications systolic and diastolic blood pressure varied in their pattern of, with the latter falling substantially (P = 0.02) while the former remained almost unchanged (P = 0.09).
As can be seen in the Figure 3, initial response to therapy began since the first month following drug administration in accordance with our protocol. Sixteen cases were shown to match partial remission criteria at the end of the third month, and one patient achieved full remission; which could first be seen at the end of the third month, gaining momentum at a sharp pace to peak at around month 21.
Outcome measures
Partial renal remission
With a mean renal remission time of 3.67 ± 2.58 months from baseline, five out of 24 patients (20.83%) reached partial renal remission.
Complete renal remission
Complete renal remission was achieved in 19 out of 24 patients (79.16%) at the end of the last follow up. Average period of complete remission was 12.45 ± 7.37 month since the beginning of treatment.
At the end of follow-up, 23 out of 24 patients (95.8%) of the patients had reached remission.
Renal relapse
Relapses occurred during the maintenance phase of therapy in 4 out of 24 patients in remission (16.6%).
Relapses happened at 15th, 18th and 21st months of therapy and all patients except one responded when retreated with cyclophosphamide pulse followed by mycophenolate mofetil.
Chronic renal failure-death
There was no refractory-to-therapy case. None of our patients developed chronic renal insufficiency or died.
Extra renal manifestation and pregnancy
The majority of the initial extra renal manifestations resolved during the maintenance phase of therapy. None of our patients developed sustained amenorrhea. During this treatment course, we had four patients who had been pregnant at 8th, 10th, 15th and 21th months since treatment commenced, when they were in partial remission, ending in unproblematic delievery. Azathioprine replased mycophenolate mofetil throught pregnancy period, with one relapse episode following delivery.
Side effects
Side effects were observed in sixteen out of the 24 patients (66.6%) with fourteen cases of infection (58.3%).
No hemorrhagic cystitis was observed while transient gastrointestinal complications affected two patients during maintenance therapy (12%) (diarrhea, gastrointestinal discomfort and nausea).
Discussion
Mainstream therapy is primarily intended to improve renal function as well as to prevent progressive disease. Mycophenolate mofetil was approved for clinical use in lupus nephritis over the past years. Several studies have been conducted on mycophenolate mofetil for maintenance therapy of lupus nephritis (Hahn et al. 2012; Sahin et al. 2008; Houssiau et al. 2010b; Dooley et al. 2011; Chan et al. 2000, 2005; Lenz et al. 2005; Weng et al. 2010; Karim et al. 2005; Flores-Suárez 2006; Borba et al. 2006; Tse et al. 2006; Laskari et al. 2010; Elyan and Ballou 2009; Ginzler et al. 2005; Mak et al. 2009).
On the basis of anecdotal reports of success with mycophenolate mofetil in patients with lupus nephritis with a considerable likelihood for poor outcomes (Chan et al. 2000, 2005; Lenz et al. 2005; Ginzler et al. 2005), in the present study we intended to examine the safety and efficacy of MMF as maintenance therapy for proliferative lupus nephritis following a short-term induction therapy with iv CYC. Favorable response with acceptable side effects were observed in most patients.
Of the 24 patients treated based on our therapeutic protocol, partial as well as full remission could be achieved in a significant percentage, 95.8% of the patients, while relapse rates were as low as 16.6%. Our study was free of severe complications such as renal failure and mortality. We had the acceptable rate of relapse, 16.6% after the 15th month, during the course of therapy, all of whom treated with short-course cyclophosphamide pulse therapy and mycophenolate mofetil to achieved remission. All cases of relapse were seen one year following the last pulse of cyclophosphamide in the third phase of therapy when MMF dose was reduced. This may indicate that our patients might have needed a longer period of treatment in the second phase. The dose of prednisolon has also been reduced in the third phase. Relapses could have been delayed had steroid been tapered at a slower pace.
One interesting point regarded successful pregnancies among our patients thought our course of therapy. These cases were in 10th, 15thand 21st month of treatment when the patients were in partial remission. Another case was in her 8th month and in complete remission but lupus nephritis relapsed following delivery.
The response rate to MMF therapy in this study was significant and compatible with other reports. Similar to Chan et al. study on 21 and 33 patients who showed 81% and 72.7% complete remission with MMF therapy (Chan et al. 2000, 2005).
In the study of Elyan and Ballou (Elyan and Ballou 2009) on 25 patients with lupus nephritis treated with MMF, 57% and 17% of patients achieved complete remission and partial remission in order. On average it took our cases 3.6 and 12.4 months to achieve partial and full remission respectively whereas Elyan and Ballou reported an average of 8.5 month. Results of the statistical comparisons among hematuria, leukocyturia, proteinuria, C3 and C4 showed significant differences before and after treatment, with GFR and creatinine changing slightly which was similar to Elyan and Ballou study (Elyan and Ballou 2009).
As our only three cases of relapse occurred throughout the tapering phase, we may safely conclude that early reduction in steroid and mycophenolate mofetil dosage can account for them.
However, one case of relapse following delivery can also be explained owing to replacing MMF with azathioprine.
Alteration trends pertaining to laboratory finding variables during the course of treatment were also shown almost parallel with other reports findings (Laskari et al. 2010; Elyan and Ballou 2009). Ginzler EM et al. published multicenter, randomized clinical trial and showed that MMF is an appropriate alternative to CYC for the treatment of renal diseases in patients with biopsy-proven lupus nephritis (Ginzler et al. 2005). Our study was in agreement with their observations.
In Laskari et al. (2010) study from Greece, thirty-three consecutive patients with proliferative lupus nephritis received oral MMF 2 g/day as maintenance therapy for a median time of 29 months. They showed a significant improvement of all renal parameters at the end of the induction treatment as well as at the latest follow-up compared to the baseline. Renal remission achievement rate to the end of the follow-up was 73% whereas it was 54% for complete remission cases. They reported 4 (12%) patients who relapsed within 19–39 months after initial response. At the end of their follow-up, 51% of patients had reached remission. Their results were in agreement with ours except for the fact that we had fewer side effects in comparison with the Laskari et al. findings (Laskari et al. 2010).
In our clinical trial, there were only two gastrointestinal complications due to MMF which is generally reversible and in comparison with cyclophosphamide side effects, seems insubstantial. The majority of women preserved ovarian function, with four pregnancies. Bone marrow suppression was not a complication of MMF in our study. In contrast to the study by Contreras et al., in which 1 episode of chronic renal failure and 1 death due to severe infection happened, such outcomes were not observed in our cases (Contreras et al. 2004). Some advantages of this study with regard to well defined criteria for examined parameters include lower dose of MMF and long-term follow-up with regular intervals.
The present study suggests that small dose MMF combined with the shortest duration of CYC therapy may be safer than long-term use of CYC without compromising efficacy. This investigation had not been intended for evaluating the effectiveness of MMF as induction therapy in proliferative lupus nephritis. However, there have been reports highlighting the role of MMF in inducing remission (Zhu et al. 2007; Mak et al. 2009; Lee et al. 2010; Walsh et al. 2007; Hui et al. 2013). According to previous data a better outcome with MMF has been demonstrated for non-Caucasian patients (Lee et al. 2010), with considering the fact that our patients were Caucasian, the present study results may put emphasis on the benefit and safety of long term use of MMF in Caucasian race. Future large cohort study of lupus nephritis patients with well defined and strict criteria for all examined parameters such as criteria for full remission, partial remission, relapse, flare as well as the opportunity to have long period of regular follow up would establish our observations.
We were also restricted in terms of the following: As it was an open label clinical trial, there was no control group then the possibility of randomization and masking was not considerable. As continuous follow up and repeated observations of variables in different time interval was performed, so there was possibility of measurements biases due to mean reversion phenomenon. Moreover, the limited numbers of patients made it impossible to generalize our findings in Caucasian race. Other factors that limited our study were the lack of any plan for re-biopsy after course of treatment.
On the other hand, our study contain valuable data on the main concern of maintenance treatment in lupus nephritis given the long period of follow up, the clear design and regular follow up of all patients. Randomized controlled trials containing a larger group could corroborate our findings.
In sum, we concluded that mycophenolate mofetil appear to be efficacious and very safe as maintenance treatment for proliferative lupus nephritis following an intensive induction therapy with a short-course of monthly iv, endoxan pulse. That improved renal remission and reduce relapse rates as well as reduction in cyclophosphamide toxicity.
References
Arends S, Grootscholten C, Derksen RHWM, Berger SP, de Sévaux RGL, Voskuyl AE, Bijl M, Berden JH: Long-term follow-up of a randomised controlled trial of azathioprine/methylprednisolone versus cyclophosphamide in patients with proliferative lupus nephritis. Ann Rheum Dis 2012, 71(6):966-973. 10.1136/annrheumdis-2011-200384
Austin H 3rd, Klippel JH, Balow JE, Le Riche N, Steinberg AD, Plotz PH, Decker JL: Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 1986, 314(10):614. 10.1056/NEJM198603063141004
Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, Urowitz M, Fortin PR, Petri M, Barr S, Gordon C, Bae SC, Isenberg D, Zoma A, Aranow C, Dooley MA, Nived O, Sturfelt G, Steinsson K, Alarcón G, Senécal JL, Zummer M, Hanly J, Ensworth S, Pope J, Edworthy S, Rahman A, Sibley J, El-Gabalawy H, McCarthy T, et al.: Mortality in systemic lupus erythematosus. Arthritis Rheum 2006, 54(8):2550-2557. 10.1002/art.21955
Borba EF, Guedes LK, Christmann RB, Figueiredo CP, Gonçalves CR, Bonfá E: Mycophenolate mofetil is effective in reducing lupus glomerulonephritis proteinuria. Rheumatol Int 2006, 26(12):1078-1083. 10.1007/s00296-006-0142-3
Cameron JS: Lupus nephritis. J Am Soc Nephrol 1999, 10(2):413-424.
Chan TM, Li FK, Tang CSO, Wong RWS, Fang GX, Ji YL, Lau CS, Wong AK, Tong MK, Chan KW, Lai KN: Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. New Engl J Med 2000, 343(16):1156-1162. 10.1056/NEJM200010193431604
Chan TM, Tse KC, Tang CSO, Mok MY, Li FK: Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 2005, 16(4):1076-1084. 10.1681/ASN.2004080686
Contreras G, Pardo V, Leclercq B, Lenz O, Tozman E, O’Nan P, Roth D: Sequential therapies for proliferative lupus nephritis. New Engl J Med 2004, 350(10):971-980. 10.1056/NEJMoa031855
Contreras G, Pardo V, Cely C, Borja E, Hurtado A, De La Cuesta C, Iqbal K, Lenz O, Asif A, Nahar N, Leclerq B, Leon C, Schulman I, Ramirez-Seijas F, Paredes A, Cepero A, Khan T, Pachon F, Tozman E, Barreto G, Hoffman D, Almeida Suarez M, Busse JC, Esquenazi M, Esquenazi A, Garcia Mayol L, Garcia Estrada H: Factors associated with poor outcomes in patients with lupus nephritis. Lupus 2005, 14(11):890-895. 10.1191/0961203305lu2238oa
Dooley MA, Jayne D, Ginzler EM, Isenberg D, Olsen NJ, Wofsy D, Eitner F, Appel GB, Contreras G, Lisk L, Solomons N, ALMS Group: Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. New Engl J Med 2011, 365(20):1886-1895. 10.1056/NEJMoa1014460
Eickenberg S, Mickholz E, Jung E, Nofer JR, Pavenstadt HJ, Jacobi AM: Mycophenolic acid counteracts B cell proliferation and plasmablast formation in patients with systemic lupus erythematosus. Arthritis Res Ther 2012, 14(3):R110. 10.1186/ar3835
Elyan M, Ballou S: The effectiveness and safety of mycophenolate mofetil in lupus nephritis. Clin Rheumatol 2009, 28(7):835-840. 10.1007/s10067-009-1163-6
Ferrantelli A, Bono L, Tortorici C, Termini R, Giammarresi C, Rotolo U: Treatment of lupus nephritis. G Ital Nefrol 2005, 22: S27.
Flanc RS, Roberts MA, Strippoli G, Chadban SJ, Kerr PG, Atkins RC: Treatment of diffuse proliferative lupus nephritis: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2004, 43(2):197. 10.1053/j.ajkd.2003.10.012
Flores-Suárez LF: Remission of severe relapsing or persistent lupus nephritis using mycophenolate mofetil. Arch Med Res 2006, 37(1):68-73. 10.1016/j.arcmed.2005.04.011
Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, Appel GB: Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. New Engl J Med 2005, 353(21):2219-2228. 10.1056/NEJMoa043731
Gourley MF, Austin H 3rd, Scott D, Yarboro CH, Vaughan EM, Muir J, Boumpas DT, Klippel JH, Balow JE, Steinberg AD: Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med 1996, 125(7):549. 10.7326/0003-4819-125-7-199610010-00003
Grootscholten C, Ligtenberg G, Hagen E, Van den Wall BA, de Glas-Vos J, Bijl M, Assmann KJ, Bruijn JA, Weening JJ, van Houwelingen HC, Derksen RH, Berden JH: Azathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trial. Kidney Int 2006, 70(4):732-742. 10.1038/sj.ki.5001630
Grootscholten C, Bajema IM, Florquin S, Steenbergen EJ, Peutz-Kootstra CJ, Goldschmeding R, Bijl M, Hagen EC, Van Houwelingen HC, Derksen RH, Berden JH: Treatment with cyclophosphamide delays the progression of chronic lesions more effectively than does treatment with azathioprine plus methylprednisolone in patients with proliferative lupus nephritis. Arthritis Rheum 2007, 56(3):924-937. 10.1002/art.22449
Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi SI, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM: American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res 2012, 64(6):797-808. 10.1002/acr.21664
Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido ER, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: Immunosuppressive therapy in lupus nephritis: The Euro‒Lupus Nephritis Trial, a randomized trial of low-dose versus high‒dose intravenous cyclophosphamide. Arthritis Rheum 2002, 46(8):2121-2131. 10.1002/art.10461
Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, de Ramon GE, Danieli MG, Abramovicz D, Blockmans D, Cauli A, Direskeneli H, Galeazzi M, Gül A, Levy Y, Petera P, Popovic R, Petrovic R, Sinico RA, Cattaneo R, Font J, Depresseux G, Cosyns JP, Cervera R: The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010, 69(01):61-64. 10.1136/ard.2008.102533
Houssiau FA, D’Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, Fiehn C, de Ramon Garrido E, Gilboe IM, Tektonidou M, Blockmans D, Ravelingien I, le Guern V, Depresseux G, Guillevin L, Cervera R: Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis 2010, 69(12):2083-2089. 10.1136/ard.2010.131995
Hui M, Garner R, Rees F, Bavakunji R, Daniel P, Varughese S, Srikanth A, Andres M, Pearce F, Leung J, Lim K, Regan M, Lanyon P: Lupus nephritis: a 15-year multi-centre experience in the UK. Lupus 2013, 22(3):328-332. 10.1177/0961203312474084
Illei GG, Austin HA, Crane M, Collins L, Gourley MF, Yarboro CH, Vaughan EM, Kuroiwa T, Danning CL, Steinberg AD, Klippel JH, Balow JE, Boumpas DT: Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med 2001, 135(4):248. 10.7326/0003-4819-135-4-200108210-00009
Ioannidis JPA, Boki KA, Katsorida ME, Drosos AA, Skopouli FN, Boletis JN, Moutsopoulos HM: Remission, relapse, and re-remission of proliferative lupus nephritis treated with cyclophosphamide. Kidney Int 2000, 57(1):258-264. 10.1046/j.1523-1755.2000.00832.x
Karim M, Pisoni C, Ferro L, Tungekar M, Abbs I, D’Cruz D, Khamashta MA, Hughes GR: Reduction of proteinuria with mycophenolate mofetil in predominantly membranous lupus nephropathy. Rheumatology 2005, 44(10):1317-1321. 10.1093/rheumatology/kei019
Korbet SM, Schwartz MM, Evans J, Lewis EJ: Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol 2007, 18(1):244-254. 10.1681/ASN.2006090992
Laskari K, Mavragani CP, Tzioufas AG, Moutsopoulos HM: Mycophenolate mofetil as maintenance therapy for proliferative lupus nephritis: a long-term observational prospective study. Arthritis Res Ther 2010, 12(6):R208. 10.1186/ar3184
Lee Y, Woo J, Choi S, Ji J, Song G: Induction and maintenance therapy for lupus nephritis: a systematic review and meta-analysis. Lupus 2010, 19(6):703-710. 10.1177/0961203309357763
Lenz O, Fornoni A, Contreras G: Defining the role of mycophenolate mofetil in the treatment of proliferative lupus nephritis. Drugs 2005, 65(17):2429-2436. 10.2165/00003495-200565170-00001
Mak A, Mok C, Chu W, To C, Wong S, Au T: Renal damage in systemic lupus erythematosus: a comparative analysis of different age groups. Lupus 2007, 16(1):28-34. 10.1177/0961203306074469
Mak A, Cheak AAC, Tan JYS, Su HC, Ho RCM, Lau CS: Mycophenolate mofetil is as efficacious as, but safer than, cyclophosphamide in the treatment of proliferative lupus nephritis: a meta-analysis and meta-regression. Rheumatology 2009, 48(8):944-952. 10.1093/rheumatology/kep120
Mok CC, Ho CT, Siu YP, Chan KW, Kwan TH, Lau CS, Wong RW, Au TC: Treatment of diffuse proliferative lupus glomerulonephritis: a comparison of two cyclophosphamide-containing regimens. Am J Kidney Dis 2001, 38(2):256. 10.1053/ajkd.2001.26084
Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G, Ghazalli R, Teo SM, Wong HS, Tan SY, Shaariah W, Tan CC, Morad Z: Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology 2005, 10(5):504-510. 10.1111/j.1440-1797.2005.00444.x
Rezaie-Yazdi Z, Forghanizadeh J, Rezaie J, Salehian P, Mansouri TH: Combination therapy with pulse cyclophosphamide plus corticosteroids improves renal outcome in patients with lupus nephritis. 2005.
Sahin GM, Sahin S, Kiziltas S, Masatlioglu S, Oguz F, Ergin H: Mycophenolate mofetil versus azathioprine in the maintenance therapy of lupus nephritis. Ren Fail 2008, 30(9):865-869. 10.1080/08860220802353843
Steinberg AD, Steinberg SC: Long-term preservation of renal function in patients with lupus nephritis receiving treatment that includes cyclophosphamide versus those treated with prednisone only. Arthritis Rheum 2005, 34(8):945-950.
Tan EM, Cohen AS, Fries JF, Masi AT, Mcshane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982, 25(11):1271-1277. 10.1002/art.1780251101
Tse K, Tang CSO, Lio W, Lam M, Chan T: Quality of life comparison between corticosteroidand-mycofenolate mofetil and corticosteroidand-oral cyclophosphamide in the treatment of severe lupus nephritis. Lupus 2006, 15(6):371-379. 10.1191/0961203306lu2307xx
Walsh M, James M, Jayne D, Tonelli M, Manns BJ, Hemmelgarn BR: Mycophenolate mofetil for induction therapy of lupus nephritis: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2007, 2(5):968-975. 10.2215/CJN.01200307
Weening JJ, D’agati V, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004, 65(2):521-530. 10.1111/j.1523-1755.2004.00443.x
Weng MY, Weng CT, Liu MF: The efficacy of low-dose mycophenolate mofetil for treatment of lupus nephritis in Taiwanese patients with systemic lupus erythematosus. Clin Rheumatol 2010, 29(7):771-775. 10.1007/s10067-010-1403-9
Zhu B, Chen N, Lin Y, Ren H, Zhang W, Wang WM, Pan X, Yu H: Mycophenolate mofetil in induction and maintenance therapy of severe lupus nephritis: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant 2007, 22(7):1933-1942. 10.1093/ndt/gfm066
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZR: Conception and design of the clinical trial, referred the patients, analyzing and interpretation the data, reading and improving the manuscript, revise the final manuscript in response to reviewers’ comments. She takes responsibility for the accuracy of study design and data analysis of this clinical trial. She has also been in charge of treatments and follow up plan. TT: Prepare of the research proposal, gathering data from the patients, writing main draft of the manuscript and revise the final version. MK: Helping in designing the clinical trial, doing statistical analysis, revise the statistical aspects of the final version of the maneuscript in accordance with reviewers’ comments. SH: Acquisition of the data and helping in drafting the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Rezaieyazdi, Z., Tavakoli, T., Khajehdaluee, M. et al. Efficacy of long-term maintenance therapy with mycophenolate mofetil in lupus nephritis. SpringerPlus 3, 638 (2014). https://doi.org/10.1186/2193-1801-3-638
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-3-638