Abstract
Introduction
This retrospective, observational study aimed to analyze and assess adherence, persistence, dosing, and use of concomitant medications of seven self-administered target drugs (abatacept, golimumab, secukinumab, tocilizumab, ustekinumab, apremilast, and tofacitinib) that are currently available in Canada for the treatment of inflammatory arthritis (IA).
Methods
We used IQVIA’s longitudinal claims databases, which include private drug plans and public plans. Patients with IA identified using a proprietary indication algorithm who initiated treatment with any of the target drugs between January 2015 and February 2019 were selected and followed for 12 months.
Results
Golimumab and apremilast had the highest proportion of patients (~ 75%) who were bio-naïve and secukinumab had the fewest bio-naïve patients (~ 43%). The oral therapies, apremilast and tofacitinib, had the lowest percentage of adherent patients (73% and 71%) followed by abatacept (83%), while the remaining drugs had adherence around 90%. Secukinumab and tofacitinib had the highest 12-month persistence rate (63% and 61%), while abatacept and apremilast had the lowest persistence rate (52% and 47%). Oral corticosteroid (OCS) use was not significantly associated with adherence. Tocilizumab, secukinumab, and ustekinumab had the highest proportion of patients (> 20%) with dose escalation at 3–4 months from index. OCS and conventional disease-modifying antirheumatic drugs (cDMARD) use decreased in post-index period across all target drugs.
Conclusion
This study identified substantial differences in patient baseline characteristics. Patients on injectable biologics were more likely to be adherent compared with those on oral drugs, possibly owing to longer dosing intervals. Other outcomes at 12 months appeared similar as evidenced by tapering of concomitant medications, although differences in persistence and dose escalation were noted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is a lack of real-world evidence in Canadian practice comparing adherence, persistence, dose escalation, and concomitant medication use between biologics and oral small-molecule therapies with differing mechanisms of action in inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis). |
This study aimed to analyze and assess adherence, persistence, dosing, and use of concomitant medications of seven self-administered target drugs with different mechanisms of action (abatacept, golimumab, secukinumab, tocilizumab, ustekinumab, apremilast, and tofacitinib) that are currently available in Canada for the treatment of inflammatory arthritis. |
What was learned from the study? |
Patients on injectable biologics were more likely to be adherent compared with oral drugs. |
Other outcomes at 12 months appeared similar, as evidenced by tapering of concomitant medications, although differences in persistence and dose escalation between treatments were noted. |
Introduction
Rheumatic diseases, which include inflammatory arthritis (IA), are a group of medical conditions characterized by pain and consequent reduction in mobility and function of one or more areas of the musculoskeletal system and include conditions such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA). RA is the most common IA in Canada, affecting 1.25% of the population [1]. IA impacts patients’ quality of life in their daily activities such as walking, personal hygiene [2], and working as well as increased fatigue and reduced sleep quality [3].
There are various treatment options for IA available in Canada. Conventional disease-modifying antirheumatic drugs (cDMARDs), such as methotrexate, sulfasalazine, hydroxychloroquine, and cyclosporine as well as oral corticosteroids (OCS) and nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used treatment options. Among the biologics, molecules targeting tumor necrosis factor alpha (TNF-α) (infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol), interleukin (IL)-6 (tocilizumab), IL-12 and IL-23 (ustekinumab), IL-23 (guselkumab), CTLA-4 (abatacept), and CD20 (rituximab) are currently available. Small molecules recently developed to target intracellular signaling are also now available, such as those that target the Janus kinase family of enzymes (tofacitinib, baricitinib, and upadacitinib) and phosphodiesterase 4 (apremilast). While these treatments are approved for use with proven efficacy, clinical response can only be achieved if the treatment is taken as prescribed and dosed appropriately. For RA specifically, studies have suggested that better adherence results in lower disease activity and improved treatment outcomes [4]. Furthermore, better adherence is also associated with healthcare cost savings [5].
A previous real-world evidence study conducted by Bhoi et al. [6] compared adherence and dosing interval of four subcutaneous (SC) anti-TNF agents (golimumab, adalimumab, etanercept, and certolizumab pegol) in patients with IA using longitudinal patient-level databases in Canada. Other prior adherence studies assessed only a limited number of treatment options within specific geographic areas in Canada, and as far as we are aware, there have been no Canadian studies on adherence and on the prevalence of dose escalation with biologics and oral small molecules with different mechanisms of action in IA [7,8,9,10]. Effective management of drugs requires a thorough understanding of their dosing patterns and the consequences of dose escalation, and there is a lack real-world evidence in Canadian practice comparing adherence, persistence, dosing, and concomitant medication use between the above-mentioned IA treatment options [11, 12]. Therefore, this study aimed to analyze and assess adherence, persistence, and dosing of seven prototypical self-administered target drugs that are currently available in Canada and have adequate capture rates in the IQVIA longitudinal claims database. We chose five subcutaneously administered biologic disease-modifying antirheumatic drugs (DMARDs) each with their own specific mechanism of action (abatacept, golimumab, secukinumab, tocilizumab, and ustekinumab) and two oral targeted synthetic DMARDs also each with a unique mechanism of action (apremilast and tofacitinib). Golimumab was chosen as a prototypical anti-TNF biologic for comparison (Sponsor decision) as it previously was found to have the highest rate of adherence among patients in Canada compared with three other subcutaneous anti-TNF biologics [6]. We examined concomitant medication use to understand comorbidities and the impact of adherence on disease outcomes.
Methods
Administrative Databases
The IQVIA longitudinal claims database used in this study drew on Canadian private and public prescription claims. Specifically, the study used prescription claims from three databases: IQVIA Private Drug Plan database (PDP), Ontario Drug Benefit database (ODB), and Régie de l'Assurance Maladie du Québec (RAMQ) [6, 13, 14]. Patients from all three sources were combined for analysis. IQVIA’s PDP database comprised drug benefit claims paid by a host of private insurers including the top ten private insurance carriers, third-party administrators, and benefit plan managers and represented approximately 83% of the total private (direct-pay) business in Canada. The ODB and RAMQ are public drug plans administered in Ontario and Quebec, respectively. The ODB database contains 100% of the fully adjudicated dispensed prescription claims from the ODB program at the anonymized patient level. IQVIA’s RAMQ dataset contains a sample of dispensed prescription claims collected at the anonymized patient level. Overall, this database represents most patients on private plans in Canada.
All sources of data were actively managed and quality controlled, and captured patient demographic characteristics, specific drugs dispensed, dosage, quantity dispensed, number of days’ supply, service date, pharmacy location, cost, payer, and prescribing physician information. Additionally, all three sources had full capture of prescriptions for a patient if the patient stayed in the drug plan. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. In accordance with our institutional policies and those of Canada, ethics approval and informed consent were not required since this is a prescription claims-level study using anonymized data.
Cohort Definition
It is important to note that these administrative claims data do not include diagnosis. Therefore, consistent with methodologies previously adopted, indication was inferred using an established rules-based algorithm [6, 13, 15]. As shown in Table 1, the study included seven target drugs of interest for IA indications: five biologic DMARDs administered by subcutaneous injection (abatacept, golimumab, secukinumab, tocilizumab, and ustekinumab) and two oral targeted-synthetic DMARDs (apremilast and tofacitinib). A selection period from 1 January 2015 to 28 February 2019, where all these drugs were listed in public formularies, was utilized for patient selection based on eligibility criteria. The target drug that a patient initiated during the selection period was defined as the “index drug.” Similarly, the date a patient first started the index drug treatment during the selection period was defined as the “index date.” A 12-month look-back period from index date was applied to ensure patients were naïve to their respective target drug. The look-back period was also used to assess patients’ history of the target drugs and other demographic characteristics. Patients in each group were analyzed for up to 12 months from index date or until they discontinued or switched to other in-market drugs. This “analysis period” was used to analyze adherence, persistence, dosing interval, and dose optimization and concomitant medication use. Owing to the consideration of the loading phase in some drugs’ treatment regimens, the first 28 days post-index were excluded from the adherence and dosing analysis for all index drugs. Additionally, a look-forward period of 3 months following the analysis period was applied to ensure that patients were still active in the drug plan and that discontinuation was not due to missing data. For each included patient, the study period was a combination of look-back period, analysis period, and look-forward period.
Patients were included in the study if they met all entry criteria: (1) inferred indication of IA based on IQVIA’s proprietary indication algorithm; (2) naïve to the target drug during the look-back period; (3) had at least one claim of one of the target drugs within the selection period; (4) were ≥ 18 years of age on date of index claim; (5) active in the drug plan within the 12-month look-back period, 12-month analysis period, and 3-month look-forward period. Patients were excluded from the study if they met at least one of the following criteria: (1) had any claim of the index drug in the 12-month look-back period; (2) patients 18–24 years of age in Ontario with index date or analysis period between 1 January 2018 and 31 March 2019 as patients were transitioning from a pediatric to an early adult program (OHIP+). Patients did not need to be continuously acquiring the index drug throughout the 12-month analysis period to be included. A patient could be indexed on only one target drug.
Study Endpoints
The following endpoints based on each study objective were measured for each target drug separately: the proportion of patients (%) adherent to each index drug within the 12-month analysis period and the proportion of patients (%) persistent at 12 months; the dosing interval of each index drug was also analyzed in the following way to explore the frequency of dose escalation and the proportion of patients (%) whose index drug dose was increased (i.e., dose escalated) within the 12-month analysis period was assessed. Additionally, the mean and median escalated dose, dose difference, and time to dose escalation within the 12-month analysis period were assessed for all dose-escalated patients. For concomitant medication use, the presence, mean number of claims per month, and total days’ supply per month of OCS use for adherent and nonadherent groups within the 12-month analysis period was assessed, as well as the presence, mean number of claims per month, and total days’ supply per month of OCS and cDMARDs (azathioprine, cyclosporine, hydroxychloroquine, leflunomide, methotrexate, and sulfasalazine) use within each target drug group pre-index and post-index.
Variable Definitions
Biologic experience was defined as the presence of any in-market biologics in the look-back period. The proposed prior biologic experience groupings were biologic-experienced and biologic-naïve, where biologic-naïve was defined as having no claims for in-market biologics in the look-back period. Biologic-experienced patients were further categorized by biologic tier on the basis of whether one, two or three or more different biologic claims were reported in the 12-month look-back period. Polypharmacy was defined as the number of concomitant medication classes [number of EphATC (level 2) classes] patients took in the look-back period and reported in numerical categories (0–3, 4–6, 7–9, 10–12, 13+).
Adherence, commonly defined as the extent to which patients take their medications as prescribed [16], was measured using the medication possession ratio (MPR). MPR was defined as the proportion of days’ supply obtained during one episode of medication use, calculated by dividing the aggregated number of days’ supply obtained during the episode by the length of the episode, excluding the last prescription fill [6, 17]. MPR was calculated for the treatment period in which patients are continuously taking the index drug with no evidence of switching to other in-market products.
Days’ supply was calculated from the number of units in a prescription and length of treatment per unit listed in the Health Canada product monograph. Patients who scored ≥ 80% MPR were considered adherent as per the commonly accepted operational definition of adherence [6, 17, 18].
Patients were considered persistent until they discontinued the index drug. Discontinuation was defined as the end of treatment of the index drug. A patient is flagged as discontinued on the index drug if (1) there is a treatment gap of more than 90 days between the end of supply of a claim and the start of a subsequent claim during the analysis period and look-forward period or (2) the patient switches to other in-market products, whichever occurred first [17].
Dosing interval was defined as the average days between units for all target drugs with SC injections (Table 1). It was estimated by taking total days on therapy and dividing by the number of units the patients received.
For the oral drugs, tofacitinib and apremilast, dosing interval was defined as average number of tablets per week.
Dose escalation was defined as having an average weekly dose that was at least 20% higher than the recommended dose [13, 19]. This could be achieved by either increasing the dose and/or shortening the dosing interval. Recommended dose(s) were based on Health Canada product monographs (Table 1). Owing to the consideration of the loading phase in some drugs’ treatment regimens, the first 28 days post-index were excluded from the analysis for all index drugs.
Statistical Analyses
Continuous variables were reported using mean, standard deviation, median, and interquartile range. Categorical variables were reported using counts and proportions. All descriptive endpoints were reported separately for each target drug. For each of the endpoints, statistical modeling was used to assess each target drug in a single model. Any patients that switched between formulations of their index drug were considered censored at their first switch of formulations. All analyses were independently performed by IQVIA using SAS 9.4 (SAS Institute, Cary, NC, USA). There was no imputation of missing data given that patients were not expected to be lost to follow-up as the 3-month look-forward ensured patients were still active in the drug plan. Where the assumptions underlying any statistical modeling were violated, appropriate alternatives were employed.
Adherence across target drugs was analyzed using a multivariate logistic regression treating adherence as a binary variable (adherent, nonadherent). Expected predictor variables in the model included target drug, age, gender, province (or group of provinces), prior biologic experience, polypharmacy, and time on treatment. Persistence was analyzed using a multivariate Cox proportional hazards model for the time to target drug treatment discontinuation. Expected predictor variables in the model included target drug, age, gender, province (or group of provinces), prior biologic experience, and polypharmacy. Patients were not expected to be lost to follow-up owing to activity requirements in the look-forward period. The proportion of patients with escalated dose of index drugs were compared across target drugs using a chi-square test. Since it is not possible to collect disease activity measures in these claims databases, post-index usage of OCS was used as a surrogate for disease flare (poor disease outcomes), and discontinuation of DMARDs and OCS was used as surrogate marker of positive disease outcomes. Concomitant OCS use was compared between adherent and nonadherent groups for each target drug. No comparisons were made across target drugs for this endpoint. The presence or absence of OCS use was assessed using a chi-square test. The number of OCS claims per patient per month and days’ supply of OCS per month were compared across all target drugs using Kruskal–Wallis test. OCS and DMARD use were compared 12 months pre-index and up to 12 months post-index for each target drug. No comparisons were made across target drugs for this endpoint. The presence or absence of OCS and DMARD use was assessed using McNemar’s test. The number of OCS and DMARD claims per patient per month and days’ supply of OCS and DMARD per month were compared across all target drugs using Wilcoxon signed-rank test.
Results
A total of 24,485 patients were initially identified. Of those, 24,410 (99.7%) were ≥ 18 years of age on the date of index claim; 20,745 (84.7%) were naïve to the target drug during the look-back period (an exception is that patients were allowed to have a maximum of one IV formulation claim of the indexed target drug as a potential loading dose in the look-back period); and 13,934 (56.9%) were active in the drug plan throughout the study period. Finally, after removal of patients aged ≤ 24 years old in Ontario with index date or analysis period between 1 January 2018 and 31 March 2019, the final total number of patients was 13,863, or 56.6% of the initial cohort. The distribution of these patients among index drugs and their baseline characteristics are presented in Table 2.
The mean [standard deviation (SD)] age for the overall cohort was 55.2 (13.0) years, and 9258 patients (66.8%) were female. Patients treated with drug with a predominant RA indication (abatacept, tocilizumab, tofacitinib) tended to be older (mean age 57.0–60.7 versus 51.2–52.4 years) with a higher proportion of female gender (73.9–77.1% versus 53.6–60.7%) and cDMARD use in the look-back period (72.8–82.6% versus 46.6–68.6%). Patients treated with either golimumab or apremilast were more likely to be bio-naïve (75.7% and 79.3%, respectively) compared with patients treated with other drugs (≤ 63.2%). Polypharmacy was a common occurrence, with 67.3% of patients taking seven or more concurrent medications in the look-back period. The proportion of patients with high polypharmacy (13+ concurrent drugs) was highest in patients treated with abatacept (30.9%), ustekinumab (26.3%), and tocilizumab (24.5%). OCS use in the look-back period was highest in patients treated with abatacept, tocilizumab, and tofacitinib (51.6–57.5%) and lowest in patients treated with secukinumab, ustekinumab, and apremilast (24.6–25.5%).
The proportion of adherent patients is presented in Table 3. Adherence, defined as an MPR ≥ 80%, was observed in 82.8–91.2% of patients taking subcutaneous injections, while 70.8–73.0% of patients under oral therapy were classified as adherent. Multivariate logistic regression model by adherence status is presented in Table 4. Adherence was significantly associated with target drugs, with ustekinumab having the highest adherence [odds ratio (OR) 1.4660, 95% confidence interval (95% CI) 1.0801–1.9898; p = 0.0141] and tofacitinib the lowest adherence (OR 0.3540, 95% CI 0.3122–0.4014; p < 0.0001) compared with golimumab. Male gender, older age, Atlantic and Quebec provinces, and three or more prior biologic experience were also significantly associated with higher adherence. To identify potential outcomes of nonadherence, patients from each drug-treatment group were assigned as either adherent or nonadherent on the basis of their MPR ≥ 80% status. Post-index usage of OCS was used as a potential surrogate for disease flare. No difference was observed between adherent and nonadherent patients for OCS use in any drug group (data not shown).
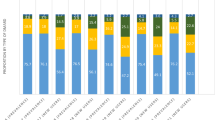
Life-table estimates of persistence rate are shown in Fig. 1. The target drugs had a 12-month persistence percentage ranging from 46.9% to 62.8%. Patients treated with apremilast had lower persistence, with 46.9% of patients on therapy after 12 months, and patients treated with secukinumab had higher persistence at 62.8%; patients on golimumab had persistence of 58.3% after 12 months. A multivariate Cox proportional hazards model suggests that persistence was significantly inversely associated with female gender [hazard ratio (HR) 1.14186, p < 0.0001], younger age (HR 1.135, p < 0.0003), provinces British Columbia and Prairies (HR 1.7072, p < 0.0001, and HR 1.427, p < 0.0001), prior biologics experience (HR 1.2992–1.5589, p < 0.0001), and higher polypharmacy (HR 1.1898–1.3048, p < 0.0004).
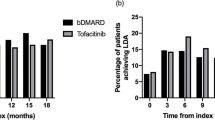
We also analyzed the frequency of dose escalation after the first 28 days post-index (Table 5). The incidence of dose escalation was lowest (≤ 6.6%) in patients taking oral therapies. Among patients taking oral therapies that dose-escalated, the percentage of dose difference from the recommended dose was also low, with a mean increase from 0.8% to 4.7%. This was expected given the higher rate of toxicity associated with higher doses. The incidence of dose escalation was more common in patients taking biologic therapies with an incidence ranging from 13.2% in patients treated with abatacept to 26.7% in patients treated with secukinumab. The lowest mean dose difference was with abatacept (5.1% increase) and highest with ustekinumab (66.1% increase). The time to dose escalation (median, IQR) was shortest with ustekinumab (29.0, 29.0–167.5 days) and longest with golimumab (116.0, 50.0–209.0 days). Drugs with multiple dosing options had variable patterns of dose escalation depending on whether patients were started at the lower or the higher dose (Table 6). Patients initiating treatment with a lower dose of tocilizumab or secukinumab experienced greater dose escalation (50.7% and 40.1%, respectively) compared with those initiating treatment on the higher dose. Indeed, the incidence of dose escalation for the lower versus higher starting dose was 40.4% versus 10.9% in tocilizumab-treated patients, and 38.5% versus 18.9% in secukinumab-treated patients, respectively. In contrast, the dose escalation pattern for ustekinumab-treated patients was the opposite, where patients treated with the higher dose (53.1%) has a higher incidence of dose escalation (31.8% versus 12.5% for the lower-dose group).
Finally, we analyzed the use of OCS and DMARDs during the pre- and post-index period to assess if the initiation of any of the target drugs are associated with tapering or discontinuation as a surrogate marker of positive disease outcomes with the target drug. As shown in Table 7, there was a significant (p < 0.0001) reduction in the proportion of patients on any dose of concomitant OCS for all drugs except for ustekinumab. With respect to the amount of OCS used, a small but significant decrease in claims and supply was observed in patients treated with abatacept, golimumab, apremilast, and tofacitinib. Patients treated with tocilizumab also had a significant reduction in the number of OCS days supplied. Discontinuation and/or dose tapering of concomitant cDMARDs was observed in all groups (Table 8).
Discussion
In the past 20 years, the launch of biologic anti-TNF therapies has revolutionized treatment of inflammatory arthritis for patients. This was followed with the development of other biologics and oral agents targeting alternative mechanism of actions (alt-MOAs) such as IL-6, IL-17, CTLA-4, IL12/23, Janus kinase, and phosphodiesterase-4. Although these alt-MOA drugs were first used predominantly following anti-TNF failure, their use as first-line therapy in patients who have failed conventional therapies (cDMARDs and/or NSAIDs) has been increasing over the years [20, 21]. Data on the comparative use of these drugs within the Canadian environment are lacking.
We selected one drug from each class of seven alt-MOA drugs listed above on the basis of the highest number of potential patients and used golimumab as a prototypical anti-TNF biologic for comparison (Sponsor decision); in a previous study of four subcutaneous anti-TNF biologics, golimumab was found to have the highest rate of adherence among patients [6]. To facilitate the comparison, we selected only drugs administered by subcutaneous injection or orally since infusion formulations of these medications are more likely to be administered by a healthcare professional in a clinical setting and, therefore, confound the study outcomes. A potential confounder is that those drugs differed by their respective indications, which could have an impact on the patient population and its baseline characteristics. Indeed, during the timeframe of this study, tocilizumab was indicated only for RA, abatacept and tofacitinib were initially indicated only for RA and then expanded to the PsA indication, ustekinumab and apremilast were indicated only for PsA, and secukinumab was indicated primarily in PsA before expanding to the AS indication, while the anti-TNF golimumab was the only drug with all three IA indications in RA, AS, and PsA. Therefore, patients treated with abatacept, tocilizumab, and tofacitinib were more likely to resemble a typical RA patient population and were likely to be older, of female gender, and with higher likelihood of concomitant cDMARD use at baseline. Both the golimumab and apremilast cohorts had a higher proportion of bio-naïve patients. This channeling bias may be driven by the long-term experience with anti-TNFs as first-line therapy, and by the lower efficacy attributes of apremilast compared with other biologic therapies [22], which makes it more likely to be used first line in patients with PsA with moderate disease activity.
All drugs appeared to have similar persistence at 12 months, except for lower persistence rate among patients on apremilast. This finding could be driven by differences in efficacy of apremilast and biologics [22] and/or patient baseline characteristics. It remains to be seen whether these persistence rates among these drugs from differing MOAs deviate over the long term.
All biologic drugs were associated with better rates of adherence compared with the two oral drugs, tofacitinib and apremilast, higher than in previous reports [5, 6, 23,24,25]. Although oral medications are deemed as more convenient and preferred by patients compared with injectable drugs [26], longer intervals between doses of medications have been strongly associated with better adherence [6, 17, 23, 27,28,29,30]. The differences in dosing interval may explain the divergence in adherence rates between injectable biologics and oral drugs reported in this study. The ease of discontinuing oral drugs in close proximity to dental surgery or fever also is a possible explanation for the divergence. The association between three or more biologics and adherence should be interpreted with caution given the low numbers of patients within each drug class ranging from 0.5% to 2.6%.
To achieve adequate clinical response, higher dose of targeted drugs may be required in some patients. In this study, we observed a large variability between the drugs with respect to dose optimization strategies. For both oral drugs, the percentage of patients with dose escalation was low, and those that did experience a dose escalation had the lowest percent increase in dose of all drugs studied. For apremilast, poor tolerability could limit the use of higher doses since the higher dose in clinical studies was associated with greater incidence of nausea and vomiting [31]. For tofacitinib, although tolerability appeared to be similar between the 5 and 10 mg doses [32], the higher dose did not receive approval from regulatory authorities owing to safety concerns around deep vein thrombosis. Additionally, dose escalation could be limited by access in some instances if payers do not reimburse the higher dose as dose escalation is an importance economic issue for healthcare payers. In the current restrictive economic environment, identification of treatments with minor need for adjusting dose may contribute to the sustainability of the health system. Among the biologics, both abatacept and golimumab were associated with a lower incidence of dose escalation relative to secukinumab, tocilizumab, and ustekinumab. Approved dose increases in the product labeling for some of these agents could result in prescribers being more comfortable with dose escalation and/or the need to achieve more optimal efficacy results. Patients treated with secukinumab, tocilizumab, and ustekinumab had the highest overall incidence of dose escalation, and these drugs were more commonly used in biologic-exposed patients. Indeed, 69.8% of tocilizumab- and 75.3% of secukinumab-treated patients were at the higher dose 12 months after the index date, either because of dose escalation or because they started at the higher dose. In contrast, ustekinumab-treated patients started at the higher dose had a much greater incidence of dose escalation, and the time to dose escalation was the shortest among all the drug cohorts. This may be the result of patient selection and channeling bias [33].
Finally, most drugs were associated with a significant proportion of patients tapering and discontinuing concomitant OCS and DMARDs in the post-index period, which could be used as surrogate for a positive outcome.
There are several limitations to this study: first, patient diagnosis was inferred using an algorithm based on patient medication history and physician specialty. The inferred indication might differ from the true diagnosis as claims are coded and recorded in the databases for payment purposes and not for research. Further, specific IA subtype (RA, AS, PsA) and disease severity were not inferred. Since some target drugs are approved for patients with specific IA subtype and/or severity level, lack of specific indication may result in confounding. Moreover, the algorithm did not allow for multiple diagnoses across other inflammatory disease conditions treated by these medications (e.g., psoriasis and inflammatory bowel disease) and, therefore, could lead to misclassification where patients have comorbid conditions. Second, patients could not be tracked across different payer plans. As a result, patients who changed plan providers could be displayed as a new patient in the plan. This limitation was mitigated by selecting patients with at least 12 months of plan history before index date and 3 months plan history in the look-forward period to ensure sufficient plan history and activity. Appropriate data cleaning processes were undertaken to ensure patients with multiple plans were deduplicated. Requiring patients to be active in the 12-month analysis period and 3-month look-forward period, however, could introduce survival bias since deceased patients were excluded from the analysis. Further, this can also cause selection bias since patients who switch plans could have different utilization patterns from patients who stayed active on the same payer plan. Third, it was not possible to collect data on disease activity or reasons for dose escalation. We measured the association of target drugs to certain outcomes of interest, such as usage of OCS and DMARDs, as a surrogate marker of disease outcomes, and there might be confounding factors that could potentially impact these outcomes. Fourth, exploration of other possible factors predictive of adherence such as psychological and patient–physician relationship was not possible given the administrative data.
Another potential limitation is that MPR does not equate with consumption of medication, but rather is only an indicator that the medication had been purchased, so this common approach provides the best possible estimate of adherence. Moreover, public plan data were collected only from the provinces of Ontario and Quebec, which might limit the generalizability of public plan findings across Canada. Lastly, there is a potential information bias due to inaccurate data entry or collection into the database by human errors. Data cleaning and quality control process were in place before the analysis, and given the large sample size, we did not anticipate a significant impact on the results due to information bias.
Despite these limitations, this is the most extensive review of adherence and dosing patterns covering seven different MOAs in Canada to date. This study used a large and nationwide sample of 13,863 patients claiming IA prescriptions in Canada. It also drew from both private and public claims databases, ensuring a wide sampling frame of Canadians. This large and representative sample of patients allowed comparison in treatment adherence, persistence, dosing, and concomitant medication use between different Alt-MOA drug groups.
The practice of dose escalation to regain or maintain clinical response is undoubtedly associated with increased drug costs and indirect IA care. Future studies should explore both the impact of dose escalation on disease activity and whether these strategies are cost effective. Effective management of drugs requires a thorough understanding of their dosing patterns and the consequences of dose escalation.
Conclusions
In summary, we observed that golimumab and apremilast had the highest proportion of patients who were bio-naïve, while other drugs are more commonly used in biologic-exposed patients. The two oral drugs, apremilast and tofacitinib, had the lowest percentage of adherent patients compared with the injectable biologics. OCS use was not significantly associated with adherence. All drugs had a similar persistence rate over 12 months except for apremilast, which was slightly lower. Tocilizumab, secukinumab, and ustekinumab had the highest proportion of patients with dose escalation, golimumab and abatacept had the lowest proportion of patients taking SC biologics with dose escalation, and the oral drugs had the lowest proportion of dose escalators of all target drugs. OCS and DMARDs use decreased in the post-index period across all target drugs, suggesting positive disease outcomes with treatment.
In conclusion, this study identified substantial differences in patient baseline characteristics among the target drugs studied. Patients on injectable biologics were more likely to be adherent compared with oral drugs, possibly owing to the longer dosing intervals of the injectable medications (weeks to months between doses) compared with the twice-daily administration of the oral medications. Other outcomes at 12 months appeared similar, as evidenced by comparative tapering of concomitant medications, although differences in persistence and dose escalation were noted.
References
Canadian Chronic Disease Surveillance System (CCDSS). https://health-infobase.canada.ca/ccdss/data-tool/. Accessed 20 July 2020.
Salaffi F, Di Carlo M, Carotti M, Farah S, Ciapetti A, Gutierrez M. The impact of different rheumatic diseases on health-related quality of life: a comparison with a selected sample of healthy individuals using SF-36 questionnaire, EQ-5D and SF-6D utility values. Acta Biomed. 2019;89(4):541–57. https://doi.org/10.23750/abm.v89i4.7298.
Katz P. Fatigue in rheumatoid arthritis. Curr Rheumatol Rep. 2017;19(5):25. https://doi.org/10.1007/s11926-017-0649-5.
Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol. 2016;43(11):1997–2009. https://doi.org/10.3899/jrheum.151212.
Lathia U, Ewara EM, Nantel F. Impact of adherence to biological agents on health care resource utilization for patients over the age of 65 years with rheumatoid arthritis. Patient Prefer Adherence. 2017;11:1133–42. https://doi.org/10.2147/ppa.s137206.
Bhoi P, Bessette L, Bell MJ, Tkaczyk C, Nantel F, Maslova K. Adherence and dosing interval of subcutaneous antitumour necrosis factor biologics among patients with inflammatory arthritis: analysis from a Canadian administrative database. BMJ Open. 2017;7(9): e015872. https://doi.org/10.1136/bmjopen-2017-015872.
Choquette D, Bessette L, Alemao E, et al. Persistence rates of abatacept and TNF inhibitors used as first or second biologic DMARDs in the treatment of rheumatoid arthritis: 9 years of experience from the Rhumadata® clinical database and registry. Arthritis Res Ther. 2019;21(1):138. https://doi.org/10.1186/s13075-019-1917-8.
Gulliver WP, Randell S, Gulliver S, Gregory V, Nagle S, Chambenoit O. Biologic therapy utilization in patients with moderate to severe psoriasis and psoriatic arthritis: an observational summary of biologic therapy use in a clinical setting. J Cutan Med Surg. 2018;22(6):567–76. https://doi.org/10.1177/1203475418786712.
Fisher A, Bassett K, Wright JM, Brookhart MA, Freeman H, Dormuth CR. Comparative persistence of the TNF antagonists in rheumatoid arthritis—a population-based cohort study. PLoS ONE. 2014;9(8): e105193. https://doi.org/10.1371/journal.pone.0105193.
Nüßlein HG, Alten R, Galeazzi M, et al. Prognostic factors for abatacept retention in patients who received at least one prior biologic agent: an interim analysis from the observational, prospective ACTION study. BMC Musculoskelet Disord. 2015;16:176. https://doi.org/10.1186/s12891-015-0636-9.
De Vera MA, Mailman J, Galo JS. Economics of non-adherence to biologic therapies in rheumatoid arthritis. Curr Rheumatol Rep. 2014;16(11):460. https://doi.org/10.1007/s11926-014-0460-5.
López-González R, León L, Loza E, Redondo M, Garcia de Yébenes MJ, Carmona L. Adherence to biologic therapies and associated factors in rheumatoid arthritis, spondyloarthritis and psoriatic arthritis: a systematic literature review. Clin Exp Rheumatol. 2015;33(4):559–69.
Khraishi M, Millson B, Woolcott J, Jones H, Marshall L, Ruperto N. Reduction in the utilization of prednisone or methotrexate in Canadian claims data following initiation of etanercept in pediatric patients with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2019;17(1):64. https://doi.org/10.1186/s12969-019-0358-x.
Lee JK, Amin S, Erdmann M, et al. Real-world observational study on the characteristics and treatment patterns of allergic asthma patients receiving omalizumab in Canada. Patient Prefer Adherence. 2020;14:725–35. https://doi.org/10.2147/PPA.S248324.
Dobson-Belaire W, Goodfield J, Borrelli R, Liu FF, Khan ZM. Identifying psoriasis and psoriatic arthritis patients in retrospective databases when diagnosis codes are not available: a validation study comparing medication/prescriber visit-based algorithms with diagnosis codes. Value Health. 2018;21(1):110–6. https://doi.org/10.1016/j.jval.2017.06.012.
Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. https://doi.org/10.1111/j.1365-2125.2012.04167.x.
Grijalva CG, Chung CP, Arbogast PG, Stein CM, Mitchel EF Jr, Griffin MR. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care. 2007;45(10 Suppl 2):S66-76. https://doi.org/10.1097/mlr.0b013e318041384c.
Curkendall S, Patel V, Gleeson M, Campbell RS, Zagari M, Dubois R. Compliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter? Arthritis Rheum. 2008;59(10):1519–26. https://doi.org/10.1002/art.24114.
Huang X, Gu NY, Fox KM, Harrison DJ, Globe D. Comparison of methods for measuring dose escalation of the subcutaneous TNF antagonists for rheumatoid arthritis patients treated in routine clinical practice. Curr Med Res Opin. 2010;26(7):1637–45. https://doi.org/10.1185/03007995.2010.483127.
Bykerk VP, Akhavan P, Hazlewood GS, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol. 2012;39(8):1559–82. https://doi.org/10.3899/jrheum.110207.
Rohekar S, Chan J, Tse SM, et al. 2014 Update of the Canadian Rheumatology Association/spondyloarthritis research consortium of Canada treatment recommendations for the management of spondyloarthritis. Part I: principles of the management of spondyloarthritis in Canada. J Rheumatol. 2014;42(4):654–64. https://doi.org/10.3899/jrheum.141000.
Schett G. Apremilast in psoriatic arthritis. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S98-100.
Calip GS, Adimadhyam S, Xing S, Rincon JC, Lee WJ, Anguiano RH. Medication adherence and persistence over time with self-administered TNF-alpha inhibitors among young adult, middle-aged, and older patients with rheumatologic conditions. Semin Arthritis Rheum. 2017;47(2):157–64. https://doi.org/10.1016/j.semarthrit.2017.03.010.
Fautrel B, Balsa A, Van Riel P, et al. Influence of route of administration/drug formulation and other factors on adherence to treatment in rheumatoid arthritis (pain related) and dyslipidemia (non-pain related). Curr Med Res Opin. 2017;33(7):1231–46. https://doi.org/10.1080/03007995.2017.1313209.
Tkacz J, Ellis L, Bolge SC, Meyer R, Brady BL, Ruetsch C. Utilization and adherence patterns of subcutaneously administered anti-tumor necrosis factor treatment among rheumatoid arthritis patients. Clin Ther. 2014;36(5):737–47. https://doi.org/10.1016/j.clinthera.2014.02.019.
Alten R, Kruger K, Rellecke J, et al. Examining patient preferences in the treatment of rheumatoid arthritis using a discrete-choice approach. Patient Prefer Adherence. 2016;10:2217–28. https://doi.org/10.2147/ppa.s117774.
Reginster JY, Rabenda V, Neuprez A. Adherence, patient preference and dosing frequency: understanding the relationship. Bone. 2006;38(4 Suppl 1):S2-6. https://doi.org/10.1016/j.bone.2006.01.150.
Borah BJ, Huang X, Zarotsky V, Globe D. Trends in RA patients’ adherence to subcutaneous anti-TNF therapies and costs. Curr Med Res Opin. 2009;25(6):1365–77. https://doi.org/10.1185/03007990902896386.
Degli Esposti L, Sangiorgi D, Perrone V, et al. Adherence and resource use among patients treated with biologic drugs: findings from BEETLE study. ClinicoEcon Outcomes Res CEOR. 2014;6:401–7. https://doi.org/10.2147/ceor.s66338.
Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum. 2009;38(5):396–402. https://doi.org/10.1016/j.semarthrit.2008.01.011.
Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42(3):479–88. https://doi.org/10.3899/jrheum.140647.
Song GG, Lee YH. Comparison of the efficacy and safety of tofacitinib and apremilast in patients with active psoriatic arthritis: a Bayesian network meta-analysis of randomized controlled trials. Clin Drug Investig. 2019;39(5):421–8. https://doi.org/10.1007/s40261-019-00765-w.
Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10(4):577–81. https://doi.org/10.1002/sim.4780100409
Acknowledgements
Funding
This study was financed by Janssen Inc. The Rapid Service Fee was funded by Janssen Inc.
Author Contributions
F.N., J.L., B.M., S.G., and H.Y. were involved in the design of the study. J.L., B.M., S.G., H.Y., and P.B. conducted analyses. F.N. drafted the manuscript. All authors participated in data interpretation and reviewed, edited, and approved the manuscript. The authors wish to acknowledge Alexandra Raymond at Janssen Inc. for her support in the conceptualization of this study.
Disclosures
Francois Nantel retired from Janssen. Meagan Rachich, Odalis Asin-Milan, and Allen J. Lehman are employees at Janssen Inc. Francois Nantel, Meagan Rachich, and Allen J. Lehman own JNJ stocks. Juejing Ling, Brad Millson, Shane Golden, Huijuan Yang, and Purva Barot are employees at IQVIA Solutions Canada Inc. and collaborated as consultants paid by Janssen Inc.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. In accordance with our institutional policies and those of Canada, ethics approval and informed consent were not required since this is a prescription claims-level study using anonymized data.
Data Availability
IQVIA will make the data available on request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Nantel, F., Ling, J., Rachich, M. et al. Usage and Adherence of Seven Advanced Therapies with Differing Mechanisms of Action for Inflammatory Arthritis in Canada. Rheumatol Ther 9, 1399–1420 (2022). https://doi.org/10.1007/s40744-022-00485-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00485-2