Abstract
Purpose
To assess the plasma oxysterol species 7-ketocholesterol (7-Kchol) and cholestane-3β,5α,6β-triol (chol-triol) as biomarkers of oxidative stress in type 1 and type 2 diabetes mellitus (DM).
Methods
In total, 26 type 1 and 80 type 2 diabetes patients, along with 205 age- and gender-matched healthy controls, were included in this study. Oxysterols were quantified by liquid chromatography coupled with tandem mass spectrometry and N,N-dimethylglycine derivatization. Correlations between oxysterols and clinical/biochemical characteristics of the diabetes patients, and factors affecting 7-Kchol and chol-triol, were also determined.
Results
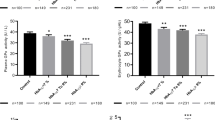
Plasma 7-Kchol and chol-triol levels were significantly higher in type 1 and type 2 diabetes patients compared to healthy controls (P < 0.001). Significant positive correlations were observed between oxysterol levels and levels of glycated hemoglobin (HbA1c), glucose, serum total cholesterol, low-density lipoprotein, very-low-density lipoprotein, and triglycerides, as well as the number of coronary risk factors. Statins, oral hypoglycemic agents, and antihypertensive agents reduced the levels of oxysterols in type 2 diabetes patients. Statin use, HbA1c levels, and the number of coronary risk factors accounted for 98.8% of the changes in 7-Kchol levels, and total cholesterol, smoking status, and the number of coronary risk factors accounted for 77.3% of the changes in chol-triol levels in type 2 diabetes patients.
Conclusions
Plasma oxysterol levels in DM, and particularly type 2 DM, may yield complementary information regarding oxidative stress for the clinical follow-up of diabetes patients, especially those with coronary risk factors.




Similar content being viewed by others
References
Rösen P, Nawroth P, King G, Möller W, Tritschler HJ, Packer L (2001) The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored byUNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev 17(3):189–212
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23(5):599–622
Inouye M, Mio T, Sumino K (1999) Glycated hemoglobin and lipid peroxidation in erythrocytes of diabetic patients. Metabolism 48(2):205–209
Iuliano L, Micheletta F, Natoli S, Corradini SG, Iappelli M, Elisei W, Giovannelli L, Violi F, Diczfalusy U (2003) Measurement of oxysterols and α-tocopherol in plasma and tissue samples as indices of oxidant stress status. Anal Biochem 312(2):217–223
Korade Z, Xu L, Mirnics K, Porter NA (2013) Lipid biomarkers of oxidative stress in a genetic mouse model of Smith-Lemli-Opitz syndrome. J Inherit Metab Dis 36(1):113–122
Arca M, Natoli S, Micheletta F, Riggi S, Di Angelantonio E, Montali A, Antonini TM, Antonini R, Diczfalusy U, Iuliano L (2007) Increased plasma levels of oxysterols, in vivo markers of oxidative stress, in patients with familial combined hyperlipidemia: reduction during atorvastatin and fenofibrate therapy. Free Radical Biol Med 42(5):698–705
Brown AJ, Jessup W (2009) Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol Aspects Med 30(3):111–122
Iuliano L (2011) Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem Phys Lipid 164(6):457–468
Vaya J, Szuchman A, Tavori H, Aluf Y (2011) Oxysterols formation as a reflection of biochemical pathways: summary of in vitro and in vivo studies. Chem Phys Lipid 164(6):438–442
Chang YH, Abdalla DS, Sevanian A (1997) Characterization of cholesterol oxidation products formed by oxidative modification of low density lipoprotein. Free Radical Biol Med 23(2):202–214
Howard J, Ingold K (1968) Self-reaction of sec-butylperoxy radicals. Confirmation of the Russell mechanism. J Am Chem Soc 90(4):1056–1058
Larsson H, Böttiger Y, Iuliano L, Diczfalusy U (2007) In vivo interconversion of 7β-hydroxycholesterol and 7-ketocholesterol, potential surrogate markers for oxidative stress. Free Radical Biol Med 43(5):695–701
Miyoshi N, Iuliano L, Tomono S, Ohshima H (2014) Implications of cholesterol autoxidation products in the pathogenesis of inflammatory diseases. Biochem Biophys Res Commun 446(3):702–708
Zarrouk A, Vejux A, Mackrill J, O’Callaghan Y, Hammami M, O’Brien N, Lizard G (2014) Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res Rev 18:148–162
Wang M, Long W, Li D, Wang D, Zhong Y, Mu D, Song J, Xia M (2017) Plasma 7-ketocholesterol levels and the risk of incident cardiovascular events. Heart 103(22):1788–1794
Deckert V, Persegol L, Viens L, Lizard G, Athias A, Lallemant C, Gambert P, Lagrost L (1997) Inhibitors of arterial relaxation among components of human oxidized low-density lipoproteins. Cholesterol derivatives oxidized in position 7 are potent inhibitors of endothelium-dependent relaxation. Circulation 95(3):723–731
Rosa-Fernandes L, Maselli LM, Maeda NY, Palmisano G, Bydlowski SP (2017) Outside-in, inside-out: proteomic analysis of endothelial stress mediated by 7-ketocholesterol. Chem Phys Lipid 207:231–238
Jessup W, Brown AJ (2005) Novel routes for metabolism of 7-ketocholesterol. Rejuvenation Res 8(1):9–12
Ferderbar S, Pereira E, Apolinario E, Bertolami M, Faludi A, Monte O, Calliari L, Sales J, Gagliardi A, Xavier H (2007) Cholesterol oxides as biomarkers of oxidative stress in type 1 and type 2 diabetes mellitus. Diabetes Metab Res Rev 23(1):35–42
Abo K, Mio T, Sumino K (2000) Comparative analysis of plasma and erythrocyte 7-ketocholesterol as a marker for oxidative stress in patients with diabetes mellitus. Clin Biochem 33(7):541–547
Yoshioka N, Adachi J, Ueno Y, Yoshida K-I (2005) Oxysterols increase in diabetic rats. Free Radical Res 39(3):299–304
Matsui H, Okumura K, Mukawa H, Hibino M, Toki Y, Ito T (1997) Increased oxysterol contents in diabetic rat hearts: their involvement in diabetic cardiomyopathy. Can J Cardiol 13(4):373–379
Endo K, Oyama T, Saiki A, Ban N, Ohira M, Koide N, Murano T, Watanabe H, Nishii M, Miura M (2008) Determination of serum 7-ketocholesterol concentrations and their relationships with coronary multiple risks in diabetes mellitus. Diabetes Res Clin Pract 80(1):63–68
Murakami H, Tamasawa N, Matsui J, Yasujima M, Suda T (2000) Plasma oxysterols and tocopherol in patients with diabetes mellitus and hyperlipidemia. Lipids 35(3):333–338
Szuchman A, Aviram M, Musa R, Khatib S, Vaya J (2008) Characterization of oxidative stress in blood from diabetic vs. hypercholesterolaemic patients, using a novel synthesized marker. Biomarkers 13(1):119–131
Association AD (2016) Standards of medical care in diabetes—2016 abridged for primary care providers. Clin Diabetes Publ Am Diabetes Assoc 34(1):3
Jiang X, Sidhu R, Porter FD, Yanjanin NM, Speak AO, te Vruchte DT, Platt FM, Fujiwara H, Scherrer DE, Zhang J (2011) A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J Lipid Res 52(7):1435–1445
Polo G, Burlina A, Furlan F, Kolamunnage T, Cananzi M, Giordano L, Zaninotto M, Plebani M, Burlina A (2016) High level of oxysterols in neonatal cholestasis: a pitfall in analysis of biochemical markers for Niemann-Pick type C disease. Clin Chem Lab Med 54(7):1221–1229
Reunert J, Lotz-Havla A, Polo G, Kannenberg F, Fobker M, Griese M, Mengel E, Muntau A, Schnabel P, Sommerburg O (2015) Niemann-Pick type C-2 disease: identification by analysis of plasma cholestane-3β, 5α, 6β-triol and further insight into the clinical phenotype. JIMD Rep 23:17–26
Meagher EA, FitzGerald GA (2000) Indices of lipid peroxidation in vivo: strengths and limitations. Free Radical Biol Med 28(12):1745–1750
Yeo HC, Helbock HJ, Chyu DW, Ames BN (1994) Assay of malondialdehyde in biological fluids by gas chromatography-mass spectrometry. Anal Biochem 220(2):391–396
Hultén LM, Lindmark H, Diczfalusy U, Björkhem I, Ottosson M, Liu Y, Bondjers G, Wiklund O (1996) Oxysterols present in atherosclerotic tissue decrease the expression of lipoprotein lipase messenger RNA in human monocyte-derived macrophages. J Clin Investig 97(2):461
Brown AJ, Dean RT, Jessup W (1996) Free and esterified oxysterol: formation during copper-oxidation of low density lipoprotein and uptake by macrophages. J Lipid Res 37(2):320–335
Smith LL (1996) Review of progress in sterol oxidations: 1987–1995. Lipids 31(5):453–487
Miyashita Y, Shirai K, Ito Y, Watanabe J, Urano Y, Murano T, Tomioka H (1997) Cytotoxicity of some oxysterols on human vascular smooth muscle cells was mediated by apoptosis. J Atheroscler Thromb 4(2):73–78
Sevanian A, Berliner J, Peterson H (1991) Uptake, metabolism, and cytotoxicity of isomeric cholesterol-5, 6-epoxides in rabbit aortic endothelial cells. J Lipid Res 32(1):147–155
Deckert V, Brunet A, Lantoine F, Lizard G, Millanvoye-van Brussel E, Monier S, Lagrost L, David-Dufilho M, Gambert P, Devynck M-A (1998) Inhibition by cholesterol oxides of NO release from human vascular endothelial cells. Arterioscler Thromb Vasc Biol 18(7):1054–1060
Tremblay-Franco M, Zerbinati C, Pacelli A, Palmaccio G, Lubrano C, Ducheix S, Guillou H, Iuliano L (2015) Effect of obesity and metabolic syndrome on plasma oxysterols and fatty acids in human. Steroids 99:287–292
Alkazemi D, Egeland G, Vaya J, Meltzer S, Kubow S (2008) Oxysterol as a marker of atherogenic dyslipidemia in adolescence. J Clin Endocrinol Metab 93(11):4282–4289
Evans JL, Maddux BA, Goldfine ID (2005) The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal 7(7–8):1040–1052
Boumhras M (2012) Evaluation de la toxicité de moules de 2 sites de la Cote Atlantique Marocaine (Jorf Lasfar et Oualidia) utilisées comme bioindicateurs de contamination: étude in vivo et in vitro sur des rats et des cellules β-pancréatiques murines (MIN-6). Ecotoxicologie. Thesis, Université de Bourgogne, Francais
Tanaka Y, Uchino H, Shimizu T, Yoshii H, Niwa M, Ohmura C, Mitsuhashi N, Onuma T, Kawamori R (1999) Effect of metformin on advanced glycation end product formation and peripheral nerve function in streptozotocin-induced diabetic rats. Eur J Pharmacol 376(1):17–22
Gargiulo P, Caccese D, Pignatelli P, Brufani C, De Vito F, Marino R, Lauro R, Violi F, Di Mario U, Sanguigni V (2002) Metformin decreases platelet superoxide anion production in diabetic patients. Diabetes Metab Res Rev 18(2):156–159
Kanigür-Sultuybek G, Güven M, Onaran İ, Tezcan V, Cenani A, Hatemi H (1995) The effect of metformin on insulin receptors and lipid peroxidation in alloxan and streptozotocin induced diabetes. J Basic Clin Physiol Pharmacol 6(3–4):271–280
Pavlović D, Kocić R, Kocić G, Jevtović T, Radenković S, Mikić D, Stojanović M, Djordjević P (2000) Effect of four-week metformin treatment on plasma and erythrocyte antioxidative defense enzymes in newly diagnosed obese patients with type 2 diabetes. Diabetes Obes Metab 2(4):251–256
Delbosc S, Morena M, Djouad F, Ledoucen C, Descomps B, Cristol J-P (2002) Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J Cardiovasc Pharmacol 40(4):611–617
Tomás M, Sentí M, García-Faria F, Vila J, Torrents A, Covas M, Marrugat J (2000) Effect of simvastatin therapy on paraoxonase activity and related lipoproteins in familial hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 20(9):2113–2119
Kaesemeyer WH, Caldwell RB, Huang J, Caldwell RW (1999) Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol 33(1):234–241
Franzoni F, Quiñones-Galvan A, Regoli F, Ferrannini E, Galetta F (2003) A comparative study of the in vitro antioxidant activity of statins. Int J Cardiol 90(2):317–321
Guan J-Z, Murakami H, Yamato K, Tanabe J, Matsui J, Tamasawa N, Suda T (2004) Effects of fluvastatin in type 2 diabetic patients with hyperlipidemia: reduction in cholesterol oxidation products and VCAM-1. J Atheroscler Thromb 11(2):56–61
Münzel T, Keaney JF (2001) Are ACE inhibitors a “magic bullet” against oxidative stress? Circulation 104(13):1571–1574
Mol MJ, de Rijke YB, Demacker PN, Stalenhoef AF (1997) Plasma levels of lipid and cholesterol oxidation products and cytokines in diabetes mellitus and cigarette smoking: effects of vitamin E treatment. Atherosclerosis 129(2):169–176
Eliasson B (2003) Cigarette smoking and diabetes. Prog Cardiovasc Dis 45(5):405–413
Funding
This study was supported by a grant from Hacettepe University Scientific Research Projects Coordination Unit (Project No: THD 201610908), Ankara, Turkey.
Author information
Authors and Affiliations
Contributions
Conceived, designed the study IL, AG. Wrote the paper IL, AS. Evaluated the patients SNS, AG. Performed the experiments AS, IL. Analyzed the data SK, AS, IL, AG, SNS. Contributed reagents/materials IL, FA.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study protocol adhered to the Declaration of Helsinki Guidelines and was approved by the Ethics Committee of Hacettepe University (GO 15/662).
Informed consent
Informed consent was obtained from each study participant.
Rights and permissions
About this article
Cite this article
Samadi, A., Gurlek, A., Sendur, S.N. et al. Oxysterol species: reliable markers of oxidative stress in diabetes mellitus. J Endocrinol Invest 42, 7–17 (2019). https://doi.org/10.1007/s40618-018-0873-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0873-5