Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental condition that includes social-communication deficits and repetitive and stereotypical behaviours (APA 2022). Neurobiological methods of studying ASD are a promising methodology for identifying ASD biomarkers. Mu rhythms (Mu) have the potential to shed light on the socialisation deficits that characterise ASD; however, Mu/ASD studies thus far have yielded inconsistent results. This review examines the existing Mu/ASD studies to determine where this variability lies to elucidate potential factors that can be addressed in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism Spectrum Disorder: What We Know
Autism spectrum disorder (ASD) is a neurodevelopmental condition affecting 1.0 to 2.9% of the population (Australian Bureau of Statistics 2014; Baio et al., 2018; Fombonne, 2018). It is characterised by persistent deficits in social communication and reciprocal social interaction, as well as the presence of rigid and repetitive behaviours, interests, and activities (American Psychiatric Association 2022). These characteristics have marked effects upon daily functioning and are associated with substantial costs over the lifetime to the autistic individual, their family unit, and the national economy (Horlin et al. 2014).
ASD is understood to have neurobiological underpinnings (Ecker, 2017; Petinou & Minaidou, 2017). Consequently, neurobiomarkers for the core symptoms are relevant for investigation (Jones & Lord, 2013). A cross-section of existing ASD neurobiomarkers includes significantly larger cerebral, cerebellar, frontal and temporal lobe, amygdala, and bilateral hippocampus volume (Courchesne et al., 2007, 2011), hyper-activation in both the right inferior frontal gyrus and the bilateral temporal regions (Wang et al., 2006), and aberrant event-related potential pathways to auditory and visual stimuli (Courchesne et al., 1985; Ferri et al., 2003).
Mu: What We Know
Mu refers to phasic electrophysiological signals that are named after their distinctively pointed negative peaks resembling the Greek letter µ (Mu) (Kropotov, 2009; Pineda, 2005). These rhythms are mostly identified in the alpha frequency band (i.e., from 8 to 13 Hz) and emanate from the sensory-motor cortex (Kropotov, 2009). To transform the Mu signal from a time-domain (when recorded by an EEG) to a frequency/power domain, a fast Fourier transformation (FFT) is commonly used, creating a number representative of Mu power (MP) (Pineda, 2005). Partial or full disappearance of MP (called ‘Mu desynchronisation’ (MD)) has been shown to occur in participants when physical movements are observed (Pineda, 2005), imagined (Gastaut & Bert, 1954), or made (Gastaut, 1952). Although Mu is independently generated in both the left and right hemispheres of the brain and can be bilaterally incoherent, the rhythms produced from the right and left hemispheres generally mirror one another (van Leeuwen et al., 1978).
In humans, Mu activity varies according to age, movement, handedness, sex, and attentional and affective state (e.g., Marshall & Meltzoff, 2011; Oberman et al., 2005; Pfurtscheller et al., 2000; Pineda, 2005; Stancák & Pfurtscheller, 1996). The summary outcomes from previous research on the effects of each of these variables in typically developing (TD) individuals are briefly reviewed below in Table 1 to provide a basis for comparison of data from Mu/ASD studies.
Due to its location over the sensorimotor cortex, most Mu research has focussed on its relation to motor activity (Pineda, 2005). However, more reports have emerged linking Mu to spatial attention and movement observation, indicating Mu is associated with multiple brain regions (Hobson & Bishop, 2017a). In a simultaneous fMRI and EEG, Yin et al., (2016) found MP to be negatively associated with neural firing in the sensorimotor network, the attention control network, and the mirror neuron system (MNS) and positively associated with neural firing in the salience network, including anterior cingulate cortex and anterior insula. MP is considered an idling state of the MNS, with higher power (or more synchronisation) when at rest (Pineda, 2005) and lower power (or desynchronisation) when activity increases in that region and neurons begin to fire. As the MNS has been identified as being a physiological correlate for Mu, this has been researched in autistic individuals.
Mu in Autistic Individuals
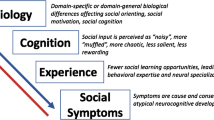
Mu rhythms (Mu) have the potential to shed light on the electrophysiological anomalies occurring in autistic individuals, particularly in relation to the core deficit of social communication and reciprocal social interaction. This is because of Mu’s overlap in functionality and topography with the mirror neuron system (MNS), which has been implicated in social functioning (Pineda, 2005). However, Mu studies in autistic individuals (Mu/ASD studies) conducted to date demonstrate some theoretical and methodological limitations and have yielded mixed results, arguing for consideration as to why such variability exists and what further research needs to be conducted to clarify the association between ASD and Mu.
Mu/ASD research began after the discovery of the MNS due to a regional and functional overlap between MD and the firing of mirror neurons (MN) (Rizzolatti & Craighero, 2004). When MN fired in synchrony, large band oscillations in Mu were produced, and these oscillations were desynchronised when movement was initiated (Pineda, 2005). Both MD and MN activation (measured by fMRI over the premotor cortex) have been shown to occur during (1) the execution (Pineda, 2005), perception (Martineau et al., 2008), and/or imagination of movement (Francuz & Zapała, 2011); (2) empathic understanding and perspective taking (Woodruff et al., 2011; Yang et al., 2009); and (3) imitative learning from language to tool use (Proverbio, 2012; Vukovic & Shtyrov, 2014). As autistic individuals often experience difficulties in empathic understanding, perspective-taking, and imitative learning (Oberman et al., 2007), unusual patterns of Mu have been investigated as potential biomarkers for ASD-related impairments. Studies investigating MD in autistic individuals have not produced consistent results, and research in this field has not previously been reviewed for a critical analysis of the consistency and validity of those results in order to clarify where the state of research is in this field and what needs to be done to provide a complete understanding of the Mu/ASD association. Therefore, the primary aim of this review is to critically analyse the previous Mu/ASD literature to determine where this variability lies and to make suggestions for reducing this variability in future studies.
Methods
To identify the existing Mu/ASD research, a search was conducted within the PubMed, Web of Science, PsycINFO, and Proquest databases, using the descriptors “Autism”, “Mu”, “Mu Rhythm”, “ASD”, “socialisation/socialization”, and “social” from the years 2005 (when the first Mu/ASD hypothesis was proposed) to 2022.
Results
A total of 14 peer-reviewed research papers incorporating an experimental paradigm were identified that met the search criteria. Table 2 provides a summary of each of these 14 papers.
Study Findings of Mu in Autistic Individuals
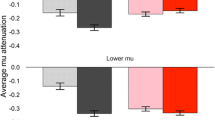
Table 2 shows the findings concerning Mu dysfunction in autistic individuals are mixed, with eight studies reporting differences between ASD and TD groups and four studies challenging these differences. Of the studies that did find significant differences in MD between ASD and TD participants, some methodological constraints have limited the generalisability of the results (Hobson & Bishop, 2017a). TD individuals desynchronise Mu under observing and/or actioning movement, so the two primary conditions included in most Mu/ASD studies were observation (e.g., observing a video of a hand performing an action) and action/imitation (e.g., copying that action). By comparing the MD in these conditions, the hypothesis that autistic individuals would fail to desynchronise Mu in the observation condition was tested. This hypothesis arose because social impairments (such as imitative ability (Pineda et al. 2008)), difficulty understanding others’ intentions (Iacoboni et al., 2005), and limitations in meaningful, goal-directed actions, that are observed in autistic individuals all must begin with focussed observation (Bernier et al., 2007; Buccino et al., 2004). Other studies included additional conditions (e.g., familiarity, genetic profiling, and face and body imitation measures prior to EEG measurement) to these two primary conditions because of earlier research that showed that these factors accounted for a proportion of the variance in MD in TD individuals (i.e., Oberman et al., 2008, 2013) The failure of autistic individuals to show MD in the observation condition was a common finding across eight of the studies (i.e., Bernier et al., 2007; Dumas et al., 2014; Hudac et al., 2015, 2017; Oberman et al., 2005, 2008, 2013), suggesting that the dysfunction of Mu occurred primarily when observing a social stimulus. However, in Dumas et al.’s (2014) study, observation condition Mu dysfunction for autistic individuals was found in the upper Mu frequency band of 10–13 Hz, but not the lower Mu band of 8–10 Hz (Dumas et al., 2014). Hudac et al. (2017) also investigated Mu in the upper (10–12 Hz) and lower bands (8–10 Hz), to create phenotypic profiles of ASD using MD attenuation and genetic profiling. The authors recruited autistic individuals with likely gene-disrupting mutations (LGDMs) and without LGDMs and then measured MD, finding those with LDGMs had more stable MD than autistic individuals without LGDMs. The concept of using MD attenuation as a phenotype for addressing some of the genetic heterogeneity in ASD was also applied in Hudac et al.’s (2015) study. This study compared MD in response to non-social movement and social movement in TD individuals (Group 1), autistic individuals with deletion (Group 2) and duplication (Group 3) copy number variants (CNVs), and autistic individuals without a CNV. They found that only the TD group showed expected MD to social stimuli, whereas the two CNV groups showed more MD for non-social movement than social movement, and the group of autistic individuals without a CNV showed very little MD across all conditions (Hudac et al., 2015). These three studies (i.e., Dumas et al., 2014, and Hudac et al., 2015, 2017) are important because their findings may partially explain the lack of consensus in the Mu/ASD studies thus far. The first reason that these studies may explain Mu/ASD inconsistencies is that two of the studies found that MD dysfunction for autistic individuals occurred in the higher Mu frequency band (10–13 Hz in Dumas et al.’s (2014) study and 10–12 Hz in Hudac et al.’s (2017) study), whereas all other Mu/ASD studies extracted Mu from 8 to 12/13 Hz. This was secondly because both Hudac et al.’s (2015, 2017) studies found the nature of the MD dysfunction varied contingent on subgroups of autistic individuals (i.e., those with and without CNV deletions and duplications and those with and without LGDMs). To address whether movement or stillness had a greater effect on MD, Martineau et al., (2008) divided the observation condition in two, viewing a human still scene (i.e., a hand not moving) versus a human movement scene (i.e., a hand moving an object). Although there were no significant differences in MD between the autistic and TD groups in the still scene condition, the autistic group failed to show MD in response to the movement scenes, whereas the TD group did. Moreover, autistic participants showed unusual activation patterns in compensatory areas of the brain, including increased cortical activity in the posterior region, centro-parietal, and temporo-occipital sites concentrated in the right hemisphere (Martineau et al., 2008). Another unusual activation pattern (i.e., slower than average EEG spectrum weighted frequency) was found over the central region of Mu in autistic participants by Pop-Jordanova et al., (2010) when they compared their ASD data to an age-matched normative database. The authors of all these studies also argued that these MD differences represented MNS dysfunction in autistic persons (Bernier et al., 2007; Dumas et al., 2014; Hudac et al., 2015, 2017; Martineau et al., 2008; Oberman et al., 2005; Pop-Jordanova et al., 2010).
The link between MD differences and MNS dysfunction in autistic individuals led to the development of two Mu/ASD neuro-feedback training (NFT) studies (Datko et al., 2018; Friedrich et al., 2015). Both studies reported that NFT was associated with emotional and behavioural improvements in autistic individuals compared to either a control group or baseline observations of the NFT group. Datko et al., (2018) provided further validity for ASD MD dysfunction by conducting an fMRI pre- and post-NFT training, which showed increased activation in the sensorimotor region in autistic participants relative to TD participants. This activation increase correlated with self and caregiver reports of behavioural improvement in the ASD group (Datko et al., 2018). While these findings seem impressive, the validity of fMRI results has been called into question (Yarkoni, 2009), with some research suggesting false-positive rates are as high as 70% (Eklund et al., 2016). This finding concerning the false-positive rate for fMRI data is more pronounced for results that show weak effect sizes. Although Datko et al., (2018) did not report effect sizes, their sample size (N = 10 ASD) was small, and their analyses were numerous (N = 11), so interpretations of their data should be treated with caution (Hobson & Bishop, 2017a; Yarkoni, 2009).
All the significant differences in MD between ASD and TD groups found in the eight studies described above (Bernier et al., 2007; Dumas et al., 2014; Hudac et al., 2015, 2017; Oberman et al., 2005, 2008, 2013) were reported to have occurred in the observation condition. However, four other studies showed that MD in the observation condition was not significantly different for autistic and TD individuals (Bernier et al., 2013; Fan et al., 2010; Raymaekers et al., 2009; Ruysschaert et al., 2014). Additionally, these studies found no significant differences in MD across any other of the conditions tested, included more participants (M = 19.25 ASD), and had greater heterogeneity in their inclusion criteria (e.g., included both sexes and different age groups).
Discussion
Mu/ASD: What Do We Need to Know?
What Causes the Inconsistency in Mu/ASD findings?
The research concerning Mu in autistic individuals cannot be considered conclusive and lacks consistency (Hobson & Bishop, 2017b). Pinpointing the factors responsible for this inconsistency can be difficult, but there are notable theoretical and methodological issues within the existing Mu/ASD studies that may be contributing to these inconsistent findings. Additionally, the six studies discussed below-measured factors that accounted for some of the variability found in the Mu/ASD studies (Bernier et al., 2013; Hudac et al., 2015, 2017; Oberman et al., 2008, 2013; Ruysschaert et al., 2014).
Possible Factors Explaining Mu Inconsistency in Previous Studies
Sample Size and Demographic Background of Participants
There are major gaps and issues in the methodology of Mu/ASD studies that need to be addressed in future studies. The ASD sample sizes of the Mu/ASD studies ranged from 10 to 20 participants, which are insufficient to achieve adequate statistical power and safeguard against false-positive and false-negative results (Hobson & Bishop, 2017a). A lack of statistical power can contribute to a reduced likelihood of detecting a true effect, but also, if a significant effect is detected, it is less likely that it is representative of a true effect (Button et al., 2013). To put this in perspective, for a study using a repeated-measures analysis of variance with two factors containing two levels (as in Bernier et al.’s (2007) study), a sample size of at least 40 participants would be required to achieve sufficient statistical power (90%) for a medium-sized main effect, and 47 would be required to detect interaction effects. Both Bernier et al., (2007) and Martineau et al., (2008) employed ASD groups of 14 participants, and Martineau included more conditions of measurement, yet both studies still reported main and interaction effects. Future Mu/ASD researchers should endeavour to increase sample sizes to provide more statistical power. In terms of demographics, Table 2 shows that the Mu/ASD study participants were either all male (in four studies) or included only minimal numbers of females in the ASD group. It has been previously established that MD varies according to the sex of the participant (e.g., Cheng et al., 2008; Yang et al., 2009), yet no Mu/ASD studies accounted for this or reported on any variation between the two sexes. Age is another demographic factor that has been investigated only infrequently in Mu/ASD studies. Age influences both MD (Hagne, 1968; Stroganova et al., 1999) and the expression of autistic traits (Padmanabhan et al., 2013), yet few studies reported on the effects of age on MD. Of those that did (i.e., Hudac et al., 2015, 2017), sample sizes were too small (N = 12), and their analyses were too many (e.g., model one N = 8, model two N = 16) to have adequate statistical power (Hobson & Bishop, 2017a). It is often difficult to find a balance between statistical power and the availability of consenting participants in sample recruitment, particularly with clinical populations. Nonetheless, given its effect in both ASD and MD, age is a factor needing increased investigation in Mu/ASD studies. To ensure adequate generalisability of the Mu/ASD studies to underrepresented subgroups of autistic individuals, Mu/ASD researchers in the future may benefit from (a) employing a larger sample size, (b) recruiting either a female-only sample or a sample with adequate numbers of males and females, and (c) including age as a factor or covariant.
Social Stimuli Used: Social Complexity and Social Familiarity
Limited generalisability in the Mu/ASD findings may be due to a lack of social relevance in the stimuli used (Oberman et al., 2007). All the Mu/ASD studies except one (i.e., Ruysschaert et al., 2014) investigated MP in relation to semi-social stimuli (e.g., a video of a moving hand or a picture of a face) and later generalised the results to socialisation performance, rather than examining Mu during actual exposure to social stimuli and social situations. A growing body of evidence suggests that the ‘quality’ of social stimuli/interactions (i.e., on-screen versus in-person) changes the neural response (Ambrus et al., 2021; Hietanen et al., 2008; Pönkänen et al., 2008, 2011). Pönkänen et al., (2008) found that viewing faces in-person evokes greater event-related potential responses than viewing a face on a screen (Ambrus et al., 2021; Hietanen et al., 2008; Pönkänen et al., 2008, 2011). In a recent EEG study, in-person interactions (i.e., brief autobiographical interactions with a researcher) were shown to elicit more robust representations of facial familiarity than repeated on-screen stimuli (e.g., the researchers’ face presented on a screen many times) (Ambrus et al., 2021). Although there have been no comparisons of on-screen and in-person conditions in the Mu/ASD studies, no significant differences were found between groups or conditions in the only Mu/ASD study with all in-person stimuli/interactions (Ruysschaert et al., 2014). This contrasts with the eight Mu/ASD studies (that used all on-screen stimuli) where differences were found between ASD and TD groups in the observation conditions. Taken together, the previous research finding differences between neural responses to on-screen and in-person stimuli and the mixed results in the Mu/ASD studies thus far indicate that Mu may also be sensitive to the ‘quality’ of the social stimuli. Controlling for a high ‘quality’, real-time social interaction and the movements involved is a challenge in an experimental study, so it is often reasonable to limit the stimuli to an on-screen presentation (Ruysschaert et al., 2014). Also, because movements trigger MD, they confound the ability to determine whether it was the social interaction or the movement that caused the MD, as well as risk movement-related artifacts in the EEG data. However, with an intentional, scripted social encounter that limits movement, it would be possible to measure MD in autistic individuals in response to real-time, generalisable, social stimuli/interactions. Embedded within the ‘quality’ of the social interactions are the two factors of social complexity and social familiarity.
Social Complexity
Mu has been reported to be sensitive to increasingly intense social stimuli in both ASD and TD individuals (Bernier et al., 2013). For example, MD was more likely to occur by viewing faces than hand movements in both ASD and TD groups (Dawson and Bernier 2007). This indicates that MD is influenced by more ‘social’ stimuli (faces contain more social information than hand gestures). Other Mu/ASD studies have found that MD varied contingent on the social complexity of the stimuli that was presented. For example, more MD would occur when viewing a hand moving a pencil than viewing a still picture of a hand (Martineau et al., 2008). This suggests that Mu may be sensitive not only to the shift of movement in the stimuli (Gastaut & Bert, 1954), but also the social complexity of the stimuli (Martineau et al., 2008).
Social Familiarity
In 2008, Oberman and colleagues found that familiarity (i.e., a familiar hand versus a novel hand) modulated MD in both autistic and TD individuals. Those authors also reported that the ASD group showed little MD in the observation condition compared to baseline and used this to support the ASD MD dysfunction hypothesis. There was no mention, however, of how the ASD group compared to the control group, which would have made a stronger argument for MD dysfunction in autistic individuals. Nonetheless, the finding that familiarity to the social stimulus impacts MD in autistic individuals may explain some of the discrepancies in the Mu/ASD studies. This is because the participants may have been more familiar with some stimuli than others (e.g., a hand cutting a piece of paper with scissors if the participant enjoyed crafts), which may have triggered more MD. Increased MD to familiar stimuli was also demonstrated in one of the earliest Mu studies by Gastaut & Bert, (1954), in which the participant showed more MD if they related to the video they were viewing (i.e., viewing a video about boxing if the person was or previously was a boxer).
Genetic Phenotyping
Two papers by Hudac et al., (2015, 2017) addressed some of the genetic mutations common in autistic individuals in relation to MD. The first paper covered deletion and duplication of CNV in autistic individuals and found that molecular subtyping could be used to form MP phenotypes. The CNV groups exhibited more MD in the non-social motion condition than the social motion condition, the ASD group without a CNV showed low levels of MD in all conditions, and the TD group showed more MD to the social motion condition (Hudac et al., 2015). Building upon their previous study, Hudac et al., (2017) used a similar paradigm to investigate MD phenotyping in ASD groups with LGDMs. Their results indicated that MD patterns could be used to differentiate between pre- and post-embryonic LGDMs, with those with the pre-embryonic LGDMs showing increased MD to social motion and those with post-embryonic LGDMs showing decreased MD to social motion. These studies are important because they show the heterogeneity of MD in autistic individuals and lend credence to the notion that MD patterns are useful phenotypic biomarkers to group autistic individuals. This MD variability within ASD groups may also help partially explain the lack of consensus in Mu/ASD studies.
Theoretical and Methodological Limitations Needing to be Addressed in Future Research
The theoretical underpinnings of the Mu/ASD studies are an important issue because the research theory drives the methodology. Most (N = 7) of the Mu/ASD studies used MD as a homologue for MNS activity and designed studies to assess ‘MNS dysfunction’ (via MD) in response to social stimuli. However, while there exists an overlap in Mu/MNS engagement (Fox et al. 2016), MD has been shown to occur in response to preparation for human movement, while MNS engagement has only been shown to occur in response to human movement (Hobson & Bishop, 2017a). So, the studies designed to assess MD function did not account for MD prior to execution because they were focussed on the function of the MNS instead. While this issue could be addressed by measuring Mu in the preparation condition, future Mu/ASD studies would benefit from building the methodology on what is known about MD, rather than the knowledge of the MNS activation.
Another reason why MNS engagement and MD should not be considered interchangeable is that the invasive nature of single-cell recording limits the opportunity to gather evidence for MN in the motor cortex of humans (where MD occurs) (Hobson & Bishop, 2017a). One of the few single-cell studies investigated the extracellular activity from 1177 cells in the medial frontal and temporal cortices of the participants’ brains while they observed and executed hand actions (Mukamel et al., 2010). Neuronal excitation occurred predominantly in the supplementary motor area and the areas surrounding the hippocampus in response to observing and executing hand actions, indicating that multiple regions of the brain were involved in mirroring functions (Mukamel et al., 2010). The authors also found neuronal subsets that were excitatory during the execution of actions and inhibitory during the observation of actions (Mukamel et al., 2010). This study indicates that the MNS in humans is more complex than in the animal models studied, and while it is probable that MD gauges an aspect of MNS function, it is not a direct measurement overlap. MD being used as a homologue for the MNS is an important conceptual limitation in Mu/ASD studies, but unfortunately, is one that is not widely considered (Pineda et al. 2008; Oberman & Ramachandran, 2007). For example, in the instances where there have been reported MD differences in the ASD groups compared to the control groups, the authors have used this as evidence for global MN dysfunction in autistic individuals (Bernier et al., 2007; Martineau et al., 2008; Oberman & Ramachandran, 2007). This is an over-simplistic conclusion and may send a deterministic message to autistic individuals, particularly those that were underrepresented in the Mu/ASD studies (e.g., ASD females and older adults). Instead, reports should focus on the Mu dysfunction itself and apply strategies for improving MD from a strong theoretical base of knowledge (Datko et al., 2018; Friedrich et al., 2015).
Additional Factors
Considering that MD is influenced by a variety of additional factors not widely addressed in the Mu/ASD studies (i.e., movement type, handedness, gender, and cognitive and affective state (see Table 1) (Gastaut & Bert, 1954; Marshall & Meltzoff, 2011; Oberman et al., 2005; Pineda, 2005; Pfurtscheller et al., 2000; Stancák & Pfurtscheller, 1996), it is clear that more research is needed investigating these factors, particularly those known to vary between autistic individuals.
Clinical Implications for Neurofeedback Training
Neurofeedback training (NFT) refers to a type of operant conditioning for neural oscillations where the participant is allowed real-time visual feedback of their neural responses (Friedrich et al., 2015). This feedback encourages the participant to gain control of their neural oscillations across cortical networks, which can result in functional and behavioural changes (Sterman and Egner 2006). Regarding Mu, NFT has been used with some success for enhancing resting MP to help facilitate MD (Friedrich et al., 2015). It has accordingly emerged as a non-invasive and effective treatment for ASD social deficits (Datko et al., 2018). In the two NFT studies reviewed in Table 2, there is evidence to suggest that Mu-specific NFT is effective at reducing ASD-related social deficits. However, the sample sizes of the NFT group in Friedrich et al.’s (2015) study were N = 6, and for Datko et al., (2018) study, N = 10, which were both too small to have sufficient statistical power to detect significant effects (Hobson & Bishop, 2017a). Additionally, in both studies, there were no follow-up assessments to determine if the social and behavioural changes were sustained.
The mixed evidence for MD dysfunction in autistic individuals indicates that the development and implementation of Mu-specific NFT programs for autistic individuals are premature. This is because NFT is designed to differentially impact brain functioning contingent on its protocol and implementation (Enriquez-Geppert et al., 2019). The importance of a strong theoretical backing prior to research or clinical implementation cannot be understated when working with the brain. There is the potential for causing harm using a protocol based on the diluted evidence for ASD MD dysfunction, which is further compounded by a lack of regulation and standards in the clinical practice of NFT (Enriquez-Geppert et al., 2019). Research that addresses the limitations of Mu/ASD studies is needed prior to developing an NFT protocol with an emphasis on Mu training.
Conclusion and Future Directions
Many of the Mu/ASD studies reviewed here point to differences between autistic and TD individuals in MP. However, important theoretical (i.e., the use of MD as a homologue for the MNS) and methodological (i.e., sample size and demographic, and social stimuli) factors limit the validity and reliability of those study’s conclusions. To ensure the Mu/ASD findings generalise to meaningful social interactions, the factors of social familiarity and complexity need to be considered during the development of the experimental stimuli. Moreover, increased knowledge about Mu variation according to gender, handedness, and cognitive and affective state in autistic individuals is also needed as these factors influence MP.
References
Aleksandrov, A. A., & Tugin, S. M. (2012). Changes in the mu rhythm in different types of motor activity and on observation of movements. Neuroscience and Behavioral Physiology, 42(3), 302–307.
Ambrus, G. G., Eick, C. M., Kaiser, D., & Kovács, G. (2021). Getting to know you: Emerging neural representations during face familiarization. Journal of Neuroscience, 41(26), 5687–5698.
Australian Bureau of Statistics. (2014). Autism in Australia, 2012 (cat. no. 4428.0). Resource document. http://www.abs.gov.au
American Psychiatric Association. (2022). Diagnostic and statistical manual of mental disorders (5th ed., text rev.). Washington, DC.
Babiloni, C., Del Percio, C., Rossini, P. M., Marzano, N., Iacoboni, M., Infarinato, F., et al. (2009). Judgment of actions in experts: A high-resolution EEG study in elite athletes. Neuroimage, 45(2), 512–521.
Baio, J., Wiggins, L., Christensen, D. L., Maenner, M. J., Daniels, J., Warren, Z., ... & Dowling, N. F. (2018). Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries, 67(6), 1–23.
Bernier, R., Dawson, G., Webb, S., & Murias, M. (2007). EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition, 64(3), 228–237.
Bernier, R., Aaronson, B., & McPartland, J. (2013). The role of imitation in the observed heterogeneity in EEG mu rhythm in autism and typical development. Brain and Cognition, 82(1), 69–75.
Buccino, G., Binkofski, F., & Riggio, L. (2004). The mirror neuron system and action recognition. Brain and Language, 89(2), 370–376.
Button, K. S., Ioannidis, J., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S., & Munafò, M. R. (2013). Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376.
Chatrian, G. E., Petersen, M. C., & Lazarte, J. A. (1959). The blocking of the rolandic wicket rhythm and some central changes related to movement. Electroencephalography and Clinical Neurophysiology, 11(3), 497–510.
Cheng, Y., Lee, P. L., Yang, C. Y., Lin, C. P., Hung, D., & Decety, J. (2008). Gender differences in the mu rhythm of the human mirror-neuron system. PLoS one, 3(5), e2113.
Courchesne, E., Lincoln, A. J., Kilman, B. A., & Galambos, R. (1985). Event-related brain potential correlates of the processing of novel visual and auditory information in autism. Journal of Autism and Developmental Disorders, 15(1), 55–76.
Courchesne, E., Pierce, K., Schumann, C. M., Redcay, E., Buckwalter, J. A., Kennedy, D. P., & Morgan, J. (2007). Mapping early brain development in autism. Neuron, 56(2), 399–413.
Courchesne, E., Campbell, K., & Solso, S. (2011). Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research, 1380, 138–145.
Datko, M., Pineda, J. A., & Müller, R. A. (2018). Positive effects of neurofeedback on autism symptoms correlate with brain activation during imitation and observation. European Journal of Neuroscience, 47(6), 579–591.
Dawson, G., & Bernier, R. (2007). Development of social brain circuitry in autism. In D. Coch, G. Dawson, & K. W Fischer (Eds.), Human behavior, learning, and the developing brain: Atypical development (pp. 28–55). The Guilford Press.
Désy, M. C., & Lepage, J. F. (2013). Skin color has no impact on motor resonance: Evidence from mu rhythm suppression and imitation. Neuroscience Research, 77(1), 58–63.
Dumas, G., Soussignan, R., Hugueville, L., Martinerie, J., & Nadel, J. (2014). Revisiting mu suppression in autism spectrum disorder. Brain Research, 1585, 108–119.
Ecker, C. (2017). The neuroanatomy of autism spectrum disorder: An overview of structural neuroimaging findings and their translatability to the clinical setting. Autism, 21(1), 18–28.
Eklund, A., Nichols, T. E., & Knutsson, H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–7905.
Enriquez-Geppert, S., Smit, D., Pimenta, M. G., & Arns, M. (2019). Neurofeedback as a treatment intervention in ADHD: Current evidence and practice. Current Psychiatry Reports, 21(6), 1–7.
Fan, Y. T., Decety, J., Yang, C. Y., Liu, J. L., & Cheng, Y. (2010). Unbroken mirror neurons in autism spectrum disorders. Journal of Child Psychology and Psychiatry, 51(9), 981–988.
Ferri, R., Elia, M., Agarwal, N., Lanuzza, B., Musumeci, S. A., & Pennisi, G. (2003). The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clinical Neurophysiology, 114(9), 1671–1680.
Fombonne, E. (2018). The rising prevalence of autism. Journal of Child Psychology and Psychiatry, 59(7), 717–720.
Fox, N. A., Bakermans-Kranenburg, M. J., Yoo, K. H., Bowman, L. C., Cannon, E. N., Vanderwert, R. E., ... & Van IJzendoorn, M. H. (2016). Assessing human mirror activity with EEG mu rhythm: A meta-analysis. Psychological Bulletin, 142(3), 291–313.
Francuz, P., & Zapała, D. (2011). The suppression of the μ rhythm during the creation of imagery representation of movement. Neuroscience Letters, 495(1), 39–43.
Friedrich, E. V., Sivanathan, A., Lim, T., Suttie, N., Louchart, S., Pillen, S., & Pineda, J. A. (2015). An effective neurofeedback intervention to improve social interactions in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(12), 4084–4100.
Gastaut, H. (1952). Electrocorticographic study of the reactivity of rolandic rhythm. Revue Neurologique, 87(2), 176–182.
Gastaut, H. J., & Bert, J. (1954). EEG changes during cinematographic presentation (Moving picture activation of the EEG). Electroencephalography and Clinical Neurophysiology, 6, 433–444.
Hagne, I. (1968). Development of the waking EEG in normal infants during the first year of life. Clinical Electroencephalography of Children, 97–118.
Hietanen, J. K., Leppänen, J. M., Peltola, M. J., Linna-Aho, K., & Ruuhiala, H. J. (2008). Seeing direct and averted gaze activates the approach–avoidance motivational brain systems. Neuropsychologia, 46(9), 2423–2430.
Hobson, H. M., & Bishop, D. V. (2016). Mu suppression–A good measure of the human mirror neuron system? Cortex, 82, 290–310.
Hobson, H. M., & Bishop, D. V. (2017). The interpretation of mu suppression as an index of mirror neuron activity: Past, present and future. Royal Society Open Science, 4(3), 160662.
Hobson, H. M., & Bishop, D. V. (2017). Reply to Bowman et al.: Building the foundations for moving mu suppression research forward. Cortex, 96, 126–128.
Horlin, C., Falkmer, M., Parsons, R., Albrecht, M. A., & Falkmer, T. (2014). The cost of autism spectrum disorders. PLoS one, 9(9), e106552.
Hudac, C. M., Kresse, A., Aaronson, B., DesChamps, T. D., Webb, S. J., & Bernier, R. A. (2015). Modulation of mu attenuation to social stimuli in children and adults with 16p11. 2 deletions and duplications. Journal of Neurodevelopmental Disorders, 7(1), 1–13.
Hudac, C. M., Stessman, H. A., DesChamps, T. D., Kresse, A., Faja, S., Neuhaus, E., ..., & Bernier, R. A. (2017). Exploring the heterogeneity of neural social indices for genetically distinct etiologies of autism. Journal of Neurodevelopmental Disorders, 9(1), 1–13
Iacoboni, M., Molnar-Szakacs, I., Gallese, V., Buccino, G., Mazziotta, J. C., & Rizzolatti, G. (2005). Grasping the intentions of others with one’s own mirror neuron system. PLoS Biology, 3(3), e79.
Jones, R. M., & Lord, C. (2013). Diagnosing autism in neurobiological research studies. Behavioural Brain Research, 251, 113–124.
Kohler, E., Keysers, C., Umilta, M. A., Fogassi, L., Gallese, V., & Rizzolatti, G. (2002). Hearing sounds, understanding actions: Action representation in mirror neurons. Science, 297(5582), 846–848.
Kropotov, J. (2009). Quantitative EEG, event related potentials and neurotherapy. Elsevier Academic Press.
Lepage, J. F., & Théoret, H. (2006). EEG evidence for the presence of an action observation–Execution matching system in children. European Journal of Neuroscience, 23(9), 2505–2510.
Li, X., Meng, X., Li, H., Yang, J., & Yuan, J. (2017). The impact of mood on empathy for pain: Evidence from an EEG study. Psychophysiology, 54(9), 1311–1322.
Marshall, P. J., & Meltzoff, A. N. (2011). Neural mirroring systems: Exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience, 1(2), 110–123.
Martineau, J., Cochin, S., Magne, R., & Barthelemy, C. (2008). Impaired cortical activation in autistic children: Is the mirror neuron system involved? International Journal of Psychophysiology, 68(1), 35–40.
Meyer, M., Hunnius, S., Van Elk, M., Van Ede, F., & Bekkering, H. (2011). Joint action modulates motor system involvement during action observation in 3-year-olds. Experimental Brain Research, 211(3), 581–592.
Moore, A., Gorodnitsky, I., & Pineda, J. (2012). EEG mu component responses to viewing emotional faces. Behavioural Brain Research, 226(1), 309–316.
Mukamel, R., Ekstrom, A. D., Kaplan, J., Iacoboni, M., & Fried, I. (2010). Single-neuron responses in humans during execution and observation of actions. Current Biology, 20(8), 750–756.
Naquet, R., & Bostem, F. (1964). Étude électroencéphalographique des syncopes. Electroencephalography and Clinical Neurophysiology, 16(1–2), 140–152.
Niedermeyer, E. (1997). Alpha rhythms as physiological and abnormal phenomena. International Journal of Psychophysiology, 26(1–3), 31–49.
Niedermeyer, E., Goldszmidt, A., & Ryan, D. (2004). “Mu rhythm status” and clinical correlates. Clinical EEG and Neuroscience, 35(2), 84–87.
Nyström, P., Ljunghammar, T., Rosander, K., & von Hofsten, C. (2011). Using mu rhythm desynchronization to measure mirror neuron activity in infants. Developmental Science, 14(2), 327–335.
Oberman, L. M., & Ramachandran, V. S. (2007). The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological Bulletin, 133(2), 310–327.
Oberman, L. M., Hubbard, E. M., McCleery, J. P., Altschuler, E. L., Ramachandran, V. S., & Pineda, J. A. (2005). EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research, 24(2), 190–198.
Oberman, L. M., Pineda, J. A., & Ramachandran, V. S. (2007). The human mirror neuron system: A link between action observation and social skills. Social Cognitive and Affective Neuroscience, 2(1), 62–66.
Oberman, L. M., Ramachandran, V. S., & Pineda, J. A. (2008). Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia, 46(5), 1558–1565.
Oberman, L. M., McCleery, J. P., Hubbard, E. M., Bernier, R., Wiersema, J. R., Raymaekers, R., & Pineda, J. A. (2013). Developmental changes in mu suppression to observed and executed actions in autism spectrum disorders. Social Cognitive and Affective Neuroscience, 8(3), 300–304.
Olfson, L. (2014). EEG study of perceptual bias in facial expressions, mood, and the mirror-neuron system.
Padmanabhan, A., Lynn, A., Foran, W., Luna, B., & O’Hearn, K. (2013). Age related changes in striatal resting state functional connectivity in autism. Frontiers in Human Neuroscience, 7(814), 1–15.
Perkins, T., Stokes, M., McGillivray, J., & Bittar, R. (2010). Mirror neuron dysfunction in autism spectrum disorders. Journal of Clinical Neuroscience, 17(10), 1239–1243.
Petinou, K., & Minaidou, D. (2017). Neurobiological bases of autism spectrum disorders and implications for early intervention: A brief overview. Folia Phoniatrica Et Logopaedica, 69(1–2), 38–42.
Pfurtscheller, G., Stancak, A., & Neuper, C. (1996). Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: A review. International Journal of Psychophysiology, 24(1), 39–46.
Pfurtscheller, G., Neuper, C., Guger, C., Harkam, W. A. H. W., Ramoser, H., Schlogl, A., et al. (2000). Current trends in Graz brain-computer interface (BCI) research. IEEE Transactions on Rehabilitation Engineering, 8(2), 216–219.
Pineda, J. A. (2005). The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing.” Brain Research Reviews, 50(1), 57–68.
Pineda, J. A., Brang, D., Hecht, E., Edwards, L., Carey, S., Bacon, M., ... & Rork, A. (2008). Positive behavioral and electrophysiological changes following neurofeedback training in children with autism. Research in Autism Spectrum Disorders, 2(3), 557–581.
Pönkänen, L. M., Hietanen, J. K., Peltola, M. J., Kauppinen, P. K., Haapalainen, A., & Leppänen, J. M. (2008). Facing a real person: An event-related potential study. Neuroreport, 19(4), 497–501.
Pönkänen, L. M., Peltola, M. J., & Hietanen, J. K. (2011). The observer observed: Frontal EEG asymmetry and autonomic responses differentiate between another person’s direct and averted gaze when the face is seen live. International Journal of Psychophysiology, 82(2), 180–187.
Pop-Jordanova, N., Zorcec, T., Demerdzieva, A., & Gucev, Z. (2010). QEEG characteristics and spectrum weighted frequency for children diagnosed as autistic spectrum disorder. Nonlinear Biomedical Physics, 4(1), 1–7.
Proverbio, A. M. (2012). Tool perception suppresses 10–12 Hz μ rhythm of EEG over the somatosensory area. Biological Psychology, 91(1), 1–7.
Raymaekers, R., Wiersema, J. R., & Roeyers, H. (2009). EEG study of the mirror neuron system in children with high functioning autism. Brain Research, 1304, 113–121.
Rizzolatti, G., & Craighero, L. (2004). The mirror-neuron system. Annual Review of Neuroscience., 27, 169–192.
Ruysschaert, L., Warreyn, P., Wiersema, J. R., Oostra, A., & Roeyers, H. (2014). Exploring the role of neural mirroring in children with autism spectrum disorder. Autism Research, 7(2), 197–206.
Singh, F., Pineda, J., & Cadenhead, K. S. (2011). Association of impaired EEG mu wave suppression, negative symptoms and social functioning in biological motion processing in first episode of psychosis. Schizophrenia Research, 130(1–3), 182–186.
Stancák, A., & Pfurtscheller, G. (1996). Event-related desynchronisation of central beta-rhythms during brisk and slow self-paced finger movements of dominant and nondominant hand. Cognitive Brain Research, 4(3), 171–183.
Stapel, J. C., Hunnius, S., van Elk, M., & Bekkering, H. (2010). Motor activation during observation of unusual versus ordinary actions in infancy. Social Neuroscience, 5(5–6), 451–460.
Sterman, M. B., & Egner, T. (2006). Foundation and practice of neurofeedback for the treatment of epilepsy. Applied Psychophysiology and Biofeedback, 31, 21–35.
Stroganova, T. A., Orekhova, E. V., & Posikera, I. N. (1999). EEG alpha rhythm in infants. Clinical Neurophysiology, 110(6), 997–1012.
van Leeuwen, W. S., Wieneke, G., Spoelstra, P., & Versteeg, H. (1978). Lack of bilateral coherence of mu rhythm. Electroencephalography and Clinical Neurophysiology, 44(2), 140–146.
Vukovic, N., & Shtyrov, Y. (2014). Cortical motor systems are involved in second-language comprehension: Evidence from rapid mu-rhythm desynchronisation. NeuroImage, 102, 695–703.
Wang, A. T., Lee, S. S., Sigman, M., & Dapretto, M. (2006). Neural basis of irony comprehension in children with autism: The role of prosody and context. Brain, 129(4), 932–943.
Woodruff, C. C., Martin, T., & Bilyk, N. (2011). Differences in self-and other-induced Mu suppression are correlated with empathic abilities. Brain Research, 1405, 69–76.
Yang, C. Y., Decety, J., Lee, S., Chen, C., & Cheng, Y. (2009). Gender differences in the mu rhythm during empathy for pain: An electroencephalographic study. Brain Research, 1251, 176–184.
Yarkoni, T. (2009). Big correlations in little studies: Inflated fMRI correlations reflect low statistical power—Commentary on Vul et al.(2009). Perspectives on Psychological Science, 4(3), 294–29.
Yin, S., Liu, Y., & Ding, M. (2016). Amplitude of sensorimotor mu rhythm is correlated with BOLD from multiple brain regions: A simultaneous EEG-fMRI study. Frontiers in Human Neuroscience, 10, 364.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lockhart, A.K., Sharpley, C.F. & Bitsika, V. Mu Desynchronisation in Autistic Individuals: What We Know and What We Need to Know. Rev J Autism Dev Disord (2023). https://doi.org/10.1007/s40489-023-00354-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40489-023-00354-w