Abstract
Purpose
Molecular imaging with 99mTc-methoxy-isobutyl-isonitrile (99mTc-MIBI, MIBI) has been used in the assessment of thyroid nodules (TNs) for more than two decades. Many studies showed that MIBI imaging is a suitable tool to rule-out malignancy when negative. However, relatively low specificity and accuracy have been described, thus, limiting its acceptance in clinical practice. Additionally, different technologies, protocols, and interpretation criteria are adopted accounting for heterogeneous data reported in the literature. Therefore, the present study was undertaken to assess the clinical use and methodology of MIBI imaging in patients with nodular thyroid disease in Europe.
Methods
A questionnaire was sent to 12 European centers of Nuclear Medicine. The questionnaire encompassed ultrasound (US) and fine-needle aspiration cytology (FNAC) procedures and their evaluation as well scintigraphy imaging indications, technical procedures, and interpretation criteria of MIBI imaging.
Results
The survey showed a good agreement of different centers in approaching TNs by TSH measurement, US evaluation and 99mTc-pertechnetate thyroid scintigraphy. MIBI imaging is mainly used to assess TNs with inconclusive/indeterminate cytological findings and selection of target nodule(s) for FNAC in patients with multi-nodular goiter. Technical procedures adopted in different centers are globally comparable and the recorded differences are unlikely to impact clinical results. However, as the main result of the present study, substantial differences were found in interpretation criteria adopted in different centers.
Conclusions
Our survey supports the urgent need of standardized interpretation criteria of thyroid MIBI imaging in order to improve its diagnostic performance and make results comparable in clinical practice.
Similar content being viewed by others
Introduction
Because of the widespread use of ultrasound (US) thyroid nodules (TNs) are a common finding, especially in currently and previously iodine-deficient countries. Although the majority of TNs are benign, non-autonomous TNs on 99mTc-pertechnetate or 123I scintigraphy may need further diagnostic work-up to exclude thyroid cancer [1, 2]. Depending on the detection of suspicious US features by using risk stratification systems (TIRADS) and the size of the nodule, fine-needle aspiration cytology (FNAC) represents the next step in this diagnostic algorithm [2,3,4]. However, in 25–40% the FNAC, results can be indeterminate or non-diagnostic [5]. Moreover, FNAC can be challenging in patients with multinodular goiter or nodules in an unfavorable location (i.e. below the thoracic aperture), and should not be performed on patients on anticoagulant therapy [6]. Molecular imaging with 99mTc-methoxy-isobutyl-isonitrile (99mTc-MIBI, MIBI) has been used in the assessment of TNs for more than two decades [7,8,9]. MIBI is a lipophilic cation and member of the isonitrile family. It accumulates within the mitochondria, which have a high negative transmembrane potential. Thus, MIBI uptake is related to high mitochondria content and increased vascularity, which are commonly seen in lung and thyroid malignant tumors as well as parathyroid adenomas [10,11,12]. Many studies showed that a MIBI negative TN has a high probability of being a benign lesion [10, 13,14,15], thus, making MIBI imaging a suitable tool to rule-out malignancy. On the other hand, relatively low specificity and accuracy have been described [6,7,8]. Selecting hypofunctioning nodules for MIBI imaging increases the overall accuracy, as hyperfunctioning nodules are commonly benign but MIBI positive due to an increased vascularization and higher proliferation rate of hyperfunctioning follicular cells. Even in hypofunctioning nodules, however, different technologies (i.e., planar and SPECT images), injected activities, protocols, and image interpretation criteria account for heterogeneous results reported in the literature [7]. Therefore, the present study was undertaken to assess the clinical use and methodology of MIBI imaging in patients with nodular thyroid disease in Europe.
Materials and methods
The study was approved by the ethics committee of Magdeburg University Hospital (No. RAD 378-32/20) and the need for informed consent was waived. Firstly, 12 nuclear medicine centers offering complete clinical management of thyroid patients (i.e., including US and FNAC in addition to nuclear imaging and therapy) agreed to participate in the present study during the EANM Thyroid Committee Interesting Group meeting (Düsseldorf 2018).
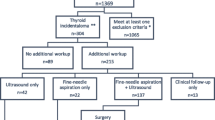
A questionnaire (Table 1) was sent to these centers in December 2018. The questionnaire encompassed US and FNAC procedures and their evaluation as well as thyroid MIBI imaging indications, technical procedures, and interpretation criteria. The survey was carried out from December 2019 until December 2020. The responses were collected by the coordinators (SAS, LG) and summarized in Tables 2, 3, 4, 5, 6, 7, 8, 9. To allow comparison, the collected qualitative/descriptive data were merged into new categories.
Results
Technical instrumentations available in the different study centers are listed in Table 2.
Initial approach in patients with thyroid nodules
Patients with TNs first underwent TSH measurement and neck US in all centers. Then, a 99mTc-pertechnetate scintigraphy was performed for nodules with a maximum diameter ≥ 10 mm and different TSH levels (any value in four centers, values from 0.50 to 3.0 mUI/L in others) (Table 3). Hyperfunctioning nodules were excluded from FNAC, while the management of hypofunctioning nodules and those not evaluated by 99mTc-pertechnetate scintigraphy (i.e., TSH above the local threshold) is centered on neck US to stratify the risk of malignancy and yardstick for additional FNAC. Among 12 participating centers, 11 (92%) use TIRADS (EU-TIRADS: eight, Kwak-TIRADS: two, ACR-TIRADS: one) for stratifying the risk of malignancy, while a descriptive report is provided by one (8%), center, respectively (Table 4). In two centers, hypofunctioning nodules larger than 10 mm underwent FNAC, one for nodules at intermediate or high risk; the other centers applied TIRADS criteria, combined with nodule’s size, for stratification. In general, FNAC is not used in nodules with a low-risk TIRADS score. Intermediate-risk nodules underwent FNAC when larger than 20 mm. Finally, high-risk nodules underwent FNAC in all cases, with some differences for nodules < 10 mm where FNAC and wait-and-see strategy are applied in different centers.
Fine-needle aspiration cytology and cytopathology reporting
Cytopathology findings are reported using the Bethesda (n = 5), SIAPEC-IAP (n = 4), German modified Bethesda (n = 2) and UK RCPath (n = 1) system respectively (Table 5).
99mTc-MIBI imaging: indications, methodology and interpretation criteria
As summarized in Table 699mTc-MIBI is mainly indicated in patients with equivocal/indeterminate FNAC results (10/12, 83%). Some differences are observed, however, in cytological subclasses selected for MIBI imaging: while most centers include all indeterminate categories (i.e., Bethesda III-IV, SIAPEC-IAP TIR3A/3B, Bethesda Germany 3, and UK RCPath Thy 3), one also includes lesions suspicious for cancer (SIAPEC-IAP TIR4), two centers only select high-risk indeterminate nodules (SIAPEC-IAP TIR 3B) and one center only selects low-risk indeterminate nodules (Bethesda III AUS/FLUS), respectively. Additional indications are non-diagnostic cytology (n = 2, 17%), multinodular goiters, to address FNAC on hypofunctioning and MIBI-positive nodules (n = 7, 58%), inapplicable FNAC (refused by patients, anticoagulant therapy and other contraindications, difficult anatomical conditions/locations) (n = 2, 17%). Finally, MIBI imaging and FNAC are simultaneously performed in one center (8%).
In all centers, 99mTc-MIBI imaging is only performed after exclusion of autonomously functioning thyroid nodules (AFTNs) using 99mTc-pertechnetate scintigraphy. Administered activities of 99mTc-MIBI ranged from 185 to 700 MBq, respectively. After tracer injection early (10–30 min) and late (60–90 min) planar images were acquired in 10 (83%) and 12 (100%) centers, respectively. An additional single-photon emission computed tomography (SPECT) is performed on a routine basis in six (50%) centers and selectively, on the decision of the attending nuclear medicine physician in two centers (16%) while it is not performed on the remaining four centers (34%) (Table 7). Two centers also perform hybrid SPECT/CT imaging in selected patients. Acquisition protocols and parameters for planar and tomographic emission imaging as well as CT imaging are summarized in Tables 7 and 8, respectively.
Finally, three different interpretation criteria are adopted in different centers: (i) comparison between 99mTc-MIBI and 99mTc-pertechnetate uptake within the nodule (four centers, 33%); (ii) comparison of 99mTc-MIBI uptake within the hypofunctioning nodule and normal thyroid tissue (six centers, 50%); (iii) semiquantitative evaluation of 99mTc-MIBI washout from the nodule (i.e., Wash-Out Index, WOI) (one center, 8%). In one center (8%) different criteria are combined (Table 9).
The concordance rate of relevant issues is summarized in Fig. 1.
Discussion
In this manuscript, the procedures, the indications, and the imaging interpretation criteria for MIBI imaging in various areas of Europe were investigated by a survey that showed a good agreement of different centers in approaching TNs by TSH measurement, US evaluation and selective use of 99mTc-pertechnetate thyroid scintigraphy based on nodule’s size (i.e., ≥ 10 mm) and TSH levels. A notable exception is observed in the German centers, where 99mTc-pertechnetate thyroid scintigraphy is performed independently of the TSH levels. Due to the fact, that the majority of AFTNs in Germany present with normal TSH levels [18], the German Society of Nuclear Medicine recommends thyroid scintigraphy for all TNs of 10 mm or larger independent of the TSH level [19]. In other centers, however, the adopted TSH thresholds are significantly higher than those proposed by clinical guidelines such as the 2015 ATA guideline reflecting differences in iodine intake and prevalence of AFTNs between the United States and Europe, as well as between different European regions. After excluding AFTNs, all but two centers based the decision to perform FNAC or not on ultrasound TIRADS patterns. Even if different US TIRADS and cytological reporting systems are adopted in different centers, significant differences are unlikely as all methods proved to be accurate and are comparable in terms of accuracy [20,21,22]. In this clinical context, 99mTc-MIBI imaging is mainly used to assess TNs with inconclusive/indeterminate cytological findings and selection target nodule(s) for FNAC in patients with multi-nodular goiter. Injected 99mTc-MIBI activities range from 185 to 700 MBq (mean 465 MBq). Early (10–30 min after intravenous injection) and late (60–90 min after intravenous injection) anterior planar images are obtained in 10 (83%) centers, while late images only are obtained in the remaining two centers (16%), respectively. An additional SPECT is also obtained as a standard in seven centers (52%) and in selected cases in two centers (16%), respectively. Hybrid SPECT/CT is also performed in two centers (16%) in selected cases (i.e., mediastinal goiters, preoperative evaluation). When performed, SPECT and SPECT/CT are performed after the late image acquisition. Visual interpretation is based on the evaluation of intranodular 99mTc-MIBI uptake compared to the normal thyroid tissue (six centers, 50%) or pertechnetate thyroid scintigraphy (four centers, 34%). Finally, a semi-quantitative assessment of WOI is employed in one (8%) center while visual and WOI are integrated in the remaining one (8%), respectively.
All in all, our present survey demonstrates a good agreement between the different nuclear thyroidology centers regarding the approach to nodular thyroid diseases. Adoption of different TIRADS systems and cytology reporting systems should be accounted as a potential source of heterogeneity. However, currently available systems are well comparable in terms of accuracy. Nonetheless, a standardization and harmonization are desirable to ameliorate communication between different specialists and allow a better comparison of research data. Technical procedures adopted in different centers are globally comparable and the recorded differences are unlikely to impact clinical results. However, as the main result of the present study, substantial differences were found in visual interpretation criteria adopted in different centers. Indeed, the WOI is only assessed in two centers for all TNs despite it has been supported as the preferred method for differentiating benign from malignant nodules and, especially, differentiate benign from malignant cytologically indeterminate nodules, respectively [10, 13]. The adoption of WOI in daily clinical practice is likely limited by the lengthening of the image analysis times and the need for strict standardization of methods. However, while a qualitatively negative 99mTc-MIBI scintigraphy reliably excludes malignancy, many benign follicular proliferation will frequently show isointense or hyperintense 99mTc-MIBI uptake and will only be discriminated by a semi-quantitative assessment. In conclusion, we found satisfactory agreement of different nuclear thyroidology centers concerning indications and technical procedures. At the same time, relevant differences exist in interpretation criteria explaining differences in diagnostic performance reported in the literature.
Preliminary data on the cost-effectiveness of 99mTc-MIBI in nodular thyroid diseases are encouraging.
Wale and colleagues evaluated the diagnostic performance of 99mTc-MIBI calculated from a retrospective review of local data on 712 patients combined with a meta-analysis of the published literature. Decision tree analysis was used to calculate the cost-effectiveness and a combined FNAC/MIBI investigative strategy was proved to be useful in avoiding unnecessary thyroidectomies, saving related costs and potential side effect [14].
Another study compared the cost-effectiveness of 99mTc-MIBI imaging and the Afirma gene expression classifier for the assessment of cytologically indeterminate TNs. Costs were calculated from the perspective of the German health insurance system. A decision tree model was used and results were confirmed by the Monte Carlo simulation. Life expectancy was 34.3 years (estimated costs per patient €1459–€2224) for the MIBI scan and 34.1 years (estimated costs €3560–€4071) for the molecular test. Therefore, the authors concluded that 99mTc-MIBI imaging is more cost-effective than the gene expression classifier [23].
However, both studies referred to local costs; then for future studies, more standardized approaches will be applied, allowing the evaluation of cost-effectiveness (99mTc-MIBI imaging combined with FNAC) in a larger and multicentric setting [14].
Conclusions
Our survey supports the urgent need for standardized interpretation criteria of thyroid 99mTc-MIBI imaging to improve its diagnostic performance and make multicenter results comparable in clinical practice. Based on this, the EANM Thyroid Committee will promote a multicenter prospective study on the clinical results of 99mTc-MIBI imaging by using harmonized 99mTc-MIBI imaging interpretation criteria, aiming to develop an EANM standardized protocol for interpreting thyroid 99mTc-MIBI imaging and improving its clinical relevance.
Data availability
Yes.
References
Campennì A, Siracusa M, Ruggeri RM et al (2017) Differentiating malignant from benign thyroid nodules with indeterminate cytology by 99mTc-MIBI scan: a new quantitative method for improving diagnostic accuracy. Sci Rep 7:6147. https://doi.org/10.1038/s41598-017-06603-3
Giovanella L, Avram AM, Iakovou I et al (2019) EANM practice guideline/SNMMI procedure standard for RAIU and thyroid scintigraphy. Eur J Nucl Med Mol Imaging 46:2514–2525. https://doi.org/10.1007/s00259-019-04472-8
Tessler FN, Middleton WD, Grant EG et al (2017) ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol 14:587–595. https://doi.org/10.1016/j.jacr.2017.01.046
Shin JH, Baek JH, Chung J et al (2016) Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol 17:370–395. https://doi.org/10.3348/kjr.2016.17.3.370
Haugen BR, Alexander EK, Bible KC et al (2015) American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133. https://doi.org/10.1089/thy.2015.0020
Yordanova A, Mahjoob S, Lingohr P et al (2017) Diagnostic accuracy of [99mTc]Tc-Sestamibi in the assessment of thyroid nodules. Oncotarget 8:94681–94691. https://doi.org/10.18632/oncotarget.21866
Schmidt M, Schenke S (2019) Update 2019 zur MIBI-Szintigrafie bei hypofunktionellen Schilddrüsenknoten. Der Nuklearmediziner 42:174–182. https://doi.org/10.1055/a-0916-6238
Treglia G, Caldarella C, Saggiorato E et al (2013) Diagnostic performance of (99m)Tc-MIBI scan in predicting the malignancy of thyroid nodules: a meta-analysis. Endocrine 44:70–78. https://doi.org/10.1007/s12020-013-9932-z
Kim S-J, Lee S-W, Jeong SY et al (2018) Diagnostic performance of Technetium-99m Methoxy-Isobutyl-Isonitrile for differentiation of Malignant Thyroid Nodules: a systematic review and meta-analysis. Thyroid 28:1339–1348. https://doi.org/10.1089/thy.2018.0072
Campennì A, Giovanella L, Siracusa M et al (2016) (99m)Tc-Methoxy-Isobutyl-Isonitrile Scintigraphy is a useful tool for Assessing the risk of Malignancy in Thyroid nodules with indeterminate fine-needle Cytology. Thyroid 26:1101–1109. https://doi.org/10.1089/thy.2016.0135
Melloul M, Paz A, Koren R et al (2001) 99mTc-MIBI scintigraphy of parathyroid adenomas and its relation to tumour size and oxyphil cell abundance. Eur J Nucl Med 28:209–213. https://doi.org/10.1007/s002590000406
Arbab AS, Koizumi K, Toyama K et al (1996) Uptake of technetium-99m-tetrofosmin, technetium-99m-MIBI and thallium-201 in tumor cell lines. J Nucl Med 37:1551–1556
Giovanella L, Campenni A, Treglia G et al (2016) Molecular imaging with (99m)Tc-MIBI and molecular testing for mutations in differentiating benign from malignant follicular neoplasm: a prospective comparison. Eur J Nucl Med Mol Imaging 43:1018–1026. https://doi.org/10.1007/s00259-015-3285-1
Wale A, Miles KA, Young B et al (2014) Combined (99m)Tc-methoxyisobutylisonitrile scintigraphy and fine-needle aspiration cytology offers an accurate and potentially cost-effective investigative strategy for the assessment of solitary or dominant thyroid nodules. Eur J Nucl Med Mol Imaging 41:105–115. https://doi.org/10.1007/s00259-013-2546-0
Schenke S, Zimny M, Rink T et al (2014) 99mTc-MIBI scintigraphy of hypofunctional thyroid nodules. Comparison of planar and SPECT imaging. Nuklearmedizin. 53:105–110. https://doi.org/10.3413/Nukmed-0619-13-08
Russ G, Bonnema SJ, Erdogan MF et al (2017) European Thyroid Association Guidelines for ultrasound malignancy risk stratification of thyroid Nodules in adults: the EU-TIRADS. Eur Thyroid J 6:225–237. https://doi.org/10.1159/000478927
Kwak JY, Han KH, Yoon JH et al (2011) Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 260:892–899. https://doi.org/10.1148/radiol.11110206
Görges R, Kandror T, Kuschnerus S et al (2011) Scintigraphically „hot“ thyroid nodules mainly go hand in hand with a normal TSH. Nuklearmedizin 50:179–188. https://doi.org/10.3413/nukmed-0386-11-02
Dietlein M, Eschner W, Lassmann M et al. Procedure Guideline for thyroid scintigraphy (Version 4) 2014. http://www.nuklearmedizin.de/leistungen/leitlinien/docs/031-011I_S1_Schiilddrüsenszintigraphie_2014-10.pdf; Accessed: 25 Mar 2021
Seifert P, Görges R, Zimny M et al (2020) Interobserver agreement and efficacy of consensus reading in Kwak-, EU-, and ACR-thyroid imaging recording and data systems and ATA guidelines for the ultrasound risk stratification of thyroid nodules. Endocrine 67:143–154. https://doi.org/10.1007/s12020-019-02134-1
Grani G, Lamartina L, Cantisani V et al (2018) Interobserver agreement of various thyroid imaging reporting and data systems. Endocr Connect 7:1–7. https://doi.org/10.1530/EC-17-0336
Grani G, Lamartina L, Ascoli V et al (2019) Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “Right” TIRADS. J Clin Endocrinol Metab 104:95–102. https://doi.org/10.1210/jc.2018-01674
Heinzel A, Müller D, Behrendt FF et al (2014) Thyroid nodules with indeterminate cytology: molecular imaging with 99mTc-methoxyisobutylisonitrile (MIBI) is more cost-effective than the Afirma gene expression classifier. Eur J Nucl Med Mol Imaging. 41:1497–500. https://doi.org/10.1007/s00259-014-2760-4
Acknowledgements
The authors would like to thank Ms. Rema Markous from Duisburg Practice of Nuclear Medicine (partly contains data from her doctoral thesis).
Author information
Authors and Affiliations
Contributions
SAS, LG: writing–original draft, formal analyses, conceptualization, methodology, investigation; AC, AP, MT, SS, TB, DR, RG, PPÖK, DG, HH, RK, MCK: investigation, writing–review, and editing, methodology.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethics approval
The study was approved by the ethics committee of Magdeburg University Hospital (No. RAD 378-32/20) and the need for an informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schenke, S.A., Campenni, A., Tuncel, M. et al. A multicenter survey of current practices of 99mTc-methoxy-isobutyl-isonitrile (MIBI) imaging for the diagnosis of thyroid nodules: more standardization is essential. Clin Transl Imaging 9, 413–422 (2021). https://doi.org/10.1007/s40336-021-00439-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-021-00439-8