Abstract
Hypertensive disorders in pregnancy are associated with increased risk of maternal, fetal, and neonatal morbidity and mortality. It is important to distinguish between pre-existing (chronic) hypertension and gestational hypertension, developing after 20 weeks of gestation and usually resolving within 6 weeks postpartum. There is a consensus that systolic blood pressure ≥ 170 or diastolic blood pressure ≥ 110 mmHg is an emergency and hospitalization is indicated. The selection of the antihypertensive drug and its route of administration depend on the expected time of delivery. The current European guidelines recommend initiating drug treatment in pregnant women with persistent elevation of blood pressure ≥ 150/95 mmHg and at values > 140/90 mmHg in women with gestational hypertension (with or without proteinuria), with pre-existing hypertension with the superimposition of gestational hypertension, and with hypertension with subclinical organ damage or symptoms at any time during pregnancy. Methyldopa, labetalol, and calcium antagonists (the most data are available for nifedipine) are the drugs of choice. The results of the CHIPS and CHAP studies are likely to reduce the threshold for initiating treatment. Women with a history of hypertensive disorders in pregnancy, particularly those with pre-eclampsia, are at high risk of developing cardiovascular disease later in life. Obstetric history should become a part of the cardiovascular risk assessment in women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hypertensive disorders in pregnancy (HDP) are a major cause of maternal, fetal, and neonatal morbidity and mortality complicating about 10% of pregnancies worldwide. This rate is likely to rise due to the increasing age and obesity of conceiving women. Pregnant women with HDP are at risk of developing placental abruption, stroke, pulmonary edema, thromboembolic events, disseminated intravascular coagulation, and multiple organ failure. The fetal risk includes intrauterine growth retardation, prematurity, and intrauterine death, all of which are particularly high in pre-eclampsia. Neonates are at increased risk of preterm birth with low birthweight, prolonged high-level neonatal care, and postnatal death [1].
2 Physiological Changes in Blood Pressure During Pregnancy
Due to vasodilation induced by local mediators such as prostacycline and nitric oxide, there is a fall in blood pressure (BP) early in the first trimester. This reduction in BP primarily affects diastolic BP (DBP), with the lowest values being achieved at weeks 20–24 (reduction of DBP by 8–15 mmHg), further followed by a gradual increase to pre-pregnancy values at week 36 [2].
This BP fluctuation is seen both in normotensive and hypertensive pregnant women. Women with pre-existing hypertension may have a greater BP decrease in early pregnancy and therefore the BP rise in the third trimester may be misdiagnosed as gestational hypertension.
Blood pressure usually falls immediately after delivery and then progressively rises over the first five postnatal days peaking on days 3–6 after delivery. It should be emphasized that 10% of maternal deaths due to hypertensive disorders in pregnancy occur in the postpartum period.
A summary of hemodynamic changes in pregnancy is provided in Table 1.
3 Blood Pressure Measurement
The initial BP measurement should be taken in both upper arms, with following measurements taken in the arm with the higher BP value, preferably in the sitting position or in the left lateral recumbent position during labor. A cuff of appropriate size should always be used with the arm being supported at heart level.
The mercury sphygmomanometer is still considered the gold standard for BP measurement in pregnancy with Korotkoff V phase to be used for DBP. However, as the sale of mercury sphygmomanometers has been banned in Europe, other devices for standard sphygmomanometry or automatic/semiautomatic (usually oscillometric) BP devices, validated according to standardized protocols (specifically for pregnancy and pre-eclampsia) should be used [3]. It is important to note that not all automatic devices are validated for use in pregnancy and pre-eclampsia. Those that are not specifically validated for this condition showed a tendency to underestimate actual BP levels and are thus unreliable in severe pre-eclampsia. A reasonable solution may be an auscultatory hybrid device with a liquid-crystal display on a vertical column simulating a mercury sphygmomanometer [4], however, these devices are not presently widely used. Wrist BP monitors are not recommended [5].
In hypertensive emergencies, BP should also be measured in both arms and in lower limbs if there is a clinical suspicion of aortic dissection [6].
Ambulatory BP monitoring (ABPM) is superior to routine BP measurement for the prediction of pregnancy outcome [7]. It can help to rule out white-coat hypertension, which is quite frequent in pregnancy [8], and may identify nocturnal hypertension, a finding commonly reported in pre-eclampsia [9].
Home BP measurement (HBPM) is suitable for long-term monitoring, particularly in patients treated with antihypertensive drugs, despite two recently published studies (BUMP 1 and BUMP 2) not showing any convincing evidence. In BUMP 1 (Blood Pressure Monitoring in High Risk Pregnancy to Improve the Detection and Monitoring of Hypertension 1) in women at high risk of pre-eclampsia, self-monitoring with telemonitoring did not lead to earlier clinic-based detection of hypertension [10]. BUMP 2, a randomized clinical trial initiated by the same group of investigators [11], did not find differences in mean systolic BP recorded by healthcare professionals in women whose BP was measured at regular antenatal clinics compared with those who performed BP self-monitoring. Together with tele-transmission of BP data, self-monitoring may become a future solution, saving repeated office visits and hospital admissions [12].
4 Diagnosis of Hypertension
Hypertension in pregnancy is diagnosed if systolic BP (SBP) ≥ 140 mmHg and/or diastolic BP (DBP) ≥ 90 mmHg, measured in the office or in hospital; it has to be confirmed, preferably on 2 separate occasions or at least 15 min apart in severe hypertension (i.e. ≥ 160/110 mmHg in the obstetric literature which usually recognizes only mild and severe hypertension rather than the three grades used by the European hypertension guidelines) [13].
5 Classification of Hypertensive Disorders
Hypertension in pregnancy is not a single entity but comprises [1, 13] (Table 2):
-
Pre-existing hypertension: either preceding pregnancy or developing before 20 weeks’ gestation. It usually persists for more than 42 days postpartum and may be associated with proteinuria.
-
Gestational hypertension: developing after 20 weeks’ gestation and usually resolving within 42 days postpartum.
-
Pre-eclampsia: gestational hypertension with significant proteinuria (> 0.3 g/24 h or ≥ 30 mg/mmol urinary protein: creatinine ratio in a spot random urine sample).
Pre-eclampsia is a systemic disorder with both maternal and fetal manifestation occurring more frequently during the first pregnancy, in multiple pregnancy, in hydatidiform mole, in antiphospholipid syndrome, in renal disease or diabetes, or with pre-existing hypertension. It is often associated with fetal growth restriction due to placental insufficiency and is a common cause of prematurity. The only cure is delivery [14]. As proteinuria may be a late manifestation of pre-eclampsia, it should be suspected when de novo hypertension is accompanied by headache, visual disturbances, abdominal pain, or abnormal laboratory tests, specifically low platelet count and abnormal liver enzymes; it is recommended to treat such patients as having pre-eclampsia.
-
Pre-existing hypertension plus superimposed gestational hypertension with proteinuria.
-
Antenatally unclassifiable hypertension: this term is used when BP is first recorded after 20 weeks' gestation and hypertension is diagnosed; re-assessment is necessary at or after 42 days postpartum.
The above definition of pre-eclampsia is in concordance with the 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy [1]. However, the International Society for the Study of Hypertension in Pregnancy (ISSHP) introduced a new, broader definition of pre-eclampsia, now being defined as gestational hypertension accompanied by one or more of the following new‐onset conditions at or after 20 weeks of gestation: (1) proteinuria; (2) evidence of other maternal organ dysfunction (including acute kidney injury [serum creatinine ≥ 1 mg/dl; 90 µ/l], liver involvement [elevated transaminases > 40 UI/L; 67 µkat/L]) with or without right upper quadrant or epigastric pain, neurological complications [convulsions, altered mental status, blindness, scotomata or headache], hematological complications [platelet count < 150,000/μL, disseminated intravascular coagulation, hemolysis]; or (3) uteroplacental dysfunction (e.g., fetal growth restrictions, abnormal umbilical artery Doppler wave form analysis for stillbirth) [15]. The combination of hemolysis, thrombocytopenia, and elevated transaminases defines HELLP syndrome as one manifestation of pre-eclampsia and therefore additional features of pre-eclampsia should be evaluated.
Pre-existing hypertension is associated with a 25% increased risk of developing superimposed pre-eclampsia [16], which is usually associated with a sharp increase in BP, and de novo development of proteinuria or any other maternal organ dysfunction as defined by the ISSHP.
The 2018 ISSHP recommendations [15] included transient gestational hypertension, which is usually detected in the clinic but settles with repeated BP measurements taken over the course of several hours. It is not a benign disorder, as it is associated with a 40% risk of developing true gestational hypertension or pre-eclampsia later in the same pregnancy. Therefore, these patients should have a careful follow-up with home BP measurements.
6 Laboratory Tests and Other Recommended Examinations
Hypertensive disorders in pregnancy, particularly gestational hypertension with or without proteinuria, may induce changes in the hematologic, renal, and hepatic profiles that may adversely affect both neonatal and maternal outcomes.
Basic laboratory investigations recommended for monitoring patients with hypertension in pregnancy are presented in Table 3. All pregnant women should be assessed for proteinuria in early pregnancy to rule out pre-existing renal disease and, in the second half of pregnancy, to screen for pre-eclampsia. A positive dipstick test (≥ 1+) should prompt further investigations including an albumin-to-creatinine ratio (ACR), which can be quickly determined in a single spot urine sample. A value < 30 mg/mmol (0.3 mg/mg) can reliably rule out proteinuria, but a positive test should possibly be followed by a 24-h urine collection. If proteinuria exceeds 2 g/day, close monitoring is warranted. It should be noted that 24-h urine collection is often inaccurate and delays the diagnosis of pre-eclampsia. Thus, an ACR cutoff of ≥ 30 mg/mmol (0.3 mg/mg) can be used to identify significant proteinuria.
The following investigations may be considered additionally to the basic laboratory tests:
-
Determination of the soluble fms-like tyrosine kinase-1 (sFlt-1) to placental growth factor (PlGF) ratio is now widely available to exclude the development of pre-eclampsia when suspected clinically [17]; a value of (sFlt-1: PlGF) < 38 is used to possibly rule out the development of pre-eclampsia in the next week. The test has a high negative predictive value. The sFlt-1/PlGF ratio is suggested to be used from 20 weeks through to 32 + 6 weeks for short-term prediction and diagnostic support in high-risk women or in women clinically suspected of pre-eclampsia [18]. It could also be used in women after 37 weeks with suspected pre-eclampsia or to evaluate uteroplacental dysfunction [19]. Women with a sFlt-1/PlGF ratio ≥ 85 most likely have or will develop pre-eclampsia within the next 4 weeks and require intensive monitoring, preferably during hospitalization [20]. A recent study showed that the increased sFlt-1/PlGF ratio in pre-eclampsia is mostly driven by the increased placental sFlt-1 [21].
-
Doppler ultrasound of uterine arteries after 20 weeks of gestation is useful in detecting women at high risk of gestational hypertension, pre-eclampsia, and intrauterine growth retardation.
-
Performing an ultrasound examination of the adrenals, urine metanephrine, and normetanephrine assays in all pregnant women with hypertension is recommended by some authors [22] as a screening for pheochromocytma.
7 Pre-conception Counselling
All women with known pre-existing hypertension should receive pre-conception counselling aimed at ruling out possible secondary causes of hypertension and informing them about the high risk of developing pre-eclampsia, which could be reduced by a low dose of aspirin [23]. Renal Doppler ultrasound is suggested to be performed in all hypertensive women planning pregnancy. If fibromuscular dysplasia (FMD) is diagnosed before pregnancy, a search for other potential arterial damage in other vascular beds should follow [24]. Determination of urine metanephrine and normetanephrine assays in all pregnant women with hypertension is recommended by some authors [22] as a screening for pheochromocytma, which may be completely asymptomatic and, if not diagnosed before labor, fatal.
8 Management of Secondary Hypertension in Pregnancy
8.1 Pheochromocytoma
During pregnancy, a pheochromocytoma is among the most life-threatening conditions for both the mother and fetus. Although extremely rare (0.002% of all pregnancies), this tumor is infamous for its devastating consequences [25]. The signs and symptoms are variable but not specific, as is the case with non-pregnant women. Hypertension is only of one of the most dominant signs. If left undiagnosed, maternal and fetal mortality is around 50%. Early detection and adequate treatment during pregnancy lower the maternal and fetal mortality to < 5 and < 15%, respectively. For the biochemical diagnosis, plasma or urinary metanephrines are the test of choice since they have the highest sensitivity and the highest negative predictive value. For reliable localization, magnetic resonance imaging is the most suitable technique, having a sensitivity of more than 90%. When a pheochromocytoma is diagnosed in pregnancy, a laparoscopic adrenalectomy should be performed after 10–14 days of drug pretreatment (as in non-pregnant patients), using alpha-adrenoreceptor blockade combined with beta-adrenergic blockade started some days later. If the pheochromocytoma is diagnosed during the third trimester, the patient should be managed until the fetus is viable using the same drug regimen as for the surgical preparation. Caesarian section with tumor removal in the same session or at a later stage is preferred, as vaginal delivery is possibly associated with higher mortality.
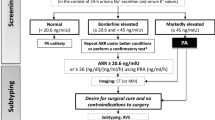
8.2 Primary Aldosteronism
Primary aldosteronism (PA), the most common cause of secondary hypertension outside of pregnancy, is underdiagnosed in pregnancy. Women with known PA before pregnancy or with clinical suspicion in early pregnancy should have a close laboratory work-up. However, optimal management of PA during pregnancy has not been established regarding the safety of mineralocorticoid antagonists and amiloride. It is also unclear if laparoscopic adrenalectomy of adrenal adenoma will improve the prognosis [26]. Eplerenone on top of the usual BP lowering treatment may be considered for uncontrolled hypertension in the second trimester [27]. Hypokalemia and BP may be aggravated postpartum due to the decrease in progesterone [26, 27].
9 Prevention of Pre-eclampsia
Women at high or moderate risk of pre-eclampsia should be advised to take 100–150 mg of aspirin daily from 12 weeks to weeks 36–37 [1, 13, 23].
High risk of pre-eclampsia includes any of the following:
-
hypertensive disease during a previous pregnancy
-
chronic kidney disease
-
autoimmune disease such as systemic lupus erythematosus or antiphospholipid syndrome
-
type 1 or type 2 diabetes
-
chronic hypertension
Moderate risk of pre-eclampsia includes ≥ 1 of the following risk factors:
-
first pregnancy
-
age 40 years or older
-
pregnancy interval of more than 10 years
-
body mass index (BMI) of 35 kg/m2 or more at first visit
-
family history of pre-eclampsia
-
multiple pregnancy.
There is growing evidence that assisted reproductive technology (ART) is associated with an increased risk of developing pre-eclampsia [28, 29]. The risk is particularly high in frozen embryo transfers [30]. Thus, it is very likely that ART in the current pregnancy will be listed as an additional high-risk condition of pre-eclampsia recommending a low dose of aspirin to prevent it.
Calcium supplementation (at least 1 g/day, orally) is recommended for prevention of pre-eclampsia in women with a low dietary intake of calcium (< 600 mg/day)[31]. Vitamin D deficiency is very common in pregnant women and increases the risk of pre-eclampsia [32]. The beneficial role of vitamin D in the prevention of pre-eclampsia, independent of timing of supplementation, dosage, and maternal age, was shown by a meta-analysis of randomized clinical trials. [33]. Further research should be focused on the recommended regimen (daily, weekly or a single dose).
There is no evidence that vitamins C and E decrease the risk of pre-eclampsia [34, 35]. On the contrary, they are associated with low birth weight (< 2.5 kg) and adverse perinatal outcomes [36].
10 Management of Hypertension in Pregnancy
The majority of women with pre-existing hypertension in pregnancy have mild to moderate hypertension (140–179/90–109 mmHg) and are at low risk for cardiovascular complications within the short timeframe of pregnancy. Women with essential hypertension and normal renal function have good maternal and neonatal outcomes. Some women with treated pre-existing, mild hypertension may be able to have their medication withdrawn or reduced in the first half of pregnancy because of the physiological fall in BP during this period. However, close monitoring and, if necessary, resumption of treatment is essential.
There are not sufficient data regarding treatment of hypertension in pregnancy as pharmaceutical companies have been reluctant to test drugs in this small market with a high potential of litigation. Child-bearing potential without reliable contraception is an exclusion criterion in basically all clinical trials testing antihypertensive drugs.
The only trial of treatment of hypertension in pregnancy with adequate infant follow-up (7.5 years) was performed almost 50 years ago with alpha-methyldopa, now rarely used in non-pregnant women [37, 38]. Past clinical trials also have not supported a beneficial effect on pregnancy outcome of treating mild to moderate hypertension. There has been no reduction in perinatal mortality, placental abruption, or superimposed pre-eclampsia [39, 40]. The most recent Cochrane Review on this topic showed only a halving of the risk of developing severe hypertension [41]. More recently, a systematic review and network meta-and trial sequential analyses found that all commonly prescribed antihypertensive drugs in pregnancy reduce the risk of severe hypertension, but labetalol may also decrease the development of proteinuria/pre-eclampsia and fetal/newborn death [42].
10.1 Non-drug Treatment
A normal diet without salt restriction is advised, particularly close to the time of delivery as salt restriction may induce a low intravascular volume. However, women with pre-existing hypertension should continue any salt-restricted diet they already follow [43].
Aerobic exercise three to four times per week (30–60 min/session) is recommended to prevent weight gain and reduce adverse pregnancy outcomes, including hypertensive disorders and gestational diabetes mellitus, unless contraindicated [44,45,46,47]. Exercise of low to moderate intensity during pregnancy is particularly effective in decreasing the development of gestational diabetes and gestational hypertension, especially when supervised and when initiated in the first trimester [48].
As maternal obesity may be associated with poor outcomes for both mother and fetus, obese women (BMI ≥ 30 kg/m2) are advised to avoid a weight gain of more than 6.8 kg. The recommended weight gain range for overweight pregnant women (BMI 25.0–29.9 kg/m2) is 6.8–11.2 kg [49,50,51].
11 Drug Treatment
The goal of treating hypertension is to reduce maternal risk without compromising the health of the fetus.
11.1 Treatment of Severe Hypertension
With values ranging between 160 and 180 mmHg/> 110 mmHg, there is no agreement on the definition of severe hypertension in pregnancy. However, there is a consensus that SBP ≥ 170 or DBP ≥ 110 mmHg in a pregnant woman should be considered an emergency, and hospitalization is indicated [1] (Table 4). The selection of the antihypertensive drug and its route of administration depend on the expected time of delivery. ACE inhibitors, ARBs, and direct renin inhibitors are strictly contraindicated.
Labetalol, methyldopa, or nifedipine XL can be used for oral treatment. If parenteral treatment is needed, intravenous labetalol seems to be the drug of choice. Intravenous hydralazine should no longer be thought of as the drug of choice, as its use is associated with more adverse effects than other drugs [52] and should only be used when labetalol is contraindicated or other drugs prove ineffective. However, recent analyses of safety and efficacy, found hydralazine to be comparable to both labetalol and nifedipine [53, 54]. Oral short-acting nifedipine should only be used temporarily, e.g. until i.v. access is available, with the second dose administered only after one hour if severe hypertension persists. Short-acting sublingual nifedipine is contraindicated.
11.2 Hypertensive Emergencies
The definition of hypertensive emergency in pregnancy is: pre-eclampsia/eclampsia and SBP ≥ 160 mmHg and DBP ≥ 110 mm Hg or severely elevated BP (DBP > 120 mmHg) and progressive acute end-organ damage such as acute myocardial infarction, pulmonary edema, respiratory failure, or aortic dissection. BP should be decreased immediately by 15–25% with the goal being SBP 140–150 mmHg and DBP 90–100 mmHg. The list of the most frequently used drugs for treatment of hypertensive emergencies is shown in Table 5.
Prolonged treatment with sodium nitroprusside is associated with an increased risk of fetal cyanide poisoning as nitroprusside is metabolized into thiocyanate excreted into urine [55]. Therefore, sodium nitroprusside should be reserved for extreme emergencies and used for the shortest possible duration.
The drug of choice in pre-eclampsia associated with pulmonary edema is nitroglycerine (given as intravenous infusion of 5 µg/min, gradually increased every 3–5 min to a maximum dose of 100 µg/min).
11.3 Prevention of Eclampsia
For prevention of eclampsia and for treatment of seizures, magnesium sulfate i.v. is recommended [56, 57]. Most guidelines suggest primary prevention of eclampsia in severe pre-eclampsia with persistent neurological symptoms (severe headache, visual disturbances, hyperactive deep-tendon reflexes). The recommended loading dose is 4 g i.v., followed by continuous infusion of 1 g per hour until delivery for a maximum of 24 h while monitoring the mother closely.
11.4 Treatment of Mild-to-Moderate Hypertension
In the absence of randomized controlled trials, recommendations can only be guided by expert opinion. The European guidelines [1, 13] recommend initiating drug treatment in all women with persistent elevation of BP ≥ 150/95 mmHg and at values > 140/90 mmHg in women with:
-
gestational hypertension (with or without proteinuria)
-
pre-existing hypertension with the superimposition of gestational hypertension
-
hypertension with subclinical organ damage or symptoms at any time during pregnancy.
Methyldopa, labetalol, and calcium antagonists (the most data are available for nifedipine) are the drugs of choice (Table 6). Beta-blockers appear to be less effective than calcium antagonists and may induce fetal bradycardia, growth retardation, and hypoglycemia; the type and dose should be carefully selected with atenolol avoided, as it was shown to be fetotoxic. Calcium-channel blockers are considered safe if not given concomitantly with magnesium sulfate (risk of hypotension due to potential synergism). Women with pre-existing hypertension may continue their current antihypertensive medication except for RAS blockers which are strictly contraindicated in pregnancy. As there is a reduction of plasma volume in pre-eclampsia, diuretic therapy is therefore inappropriate unless there is oliguria when low-dose furosemide may be considered. (Table 7). Magnesium sulfate i.v. is recommended for the prevention of eclampsia and treatment of seizures [56].
Future guidelines are likely to be influenced by two randomized clinical trials conducted in women with non-severe hypertension in pregnancy. The Control of Hypertension in Pregnancy Study (CHIPS) assessed whether “more tight” or “less tight” control of hypertension was associated with better outcomes [58]. Most women included in this study had non-severe and non-proteinuric chronic hypertension (75%, 736 out of 987) and 25% had gestational hypertension. Similar to previous meta-analyses [39, 40], the development of severe hypertension was significantly reduced in the “more tight” arm of the study. In the subgroup of women with chronic hypertension, “less tight” BP control was associated with lower rates of small-for-gestational age newborns. Unfortunately, the study has several limitations: (a) the sample size was limited, not allowing for subgroup analyses, including that of small-for-gestational age newborns; (b) chronic and gestational hypertension were analyzed together as one group; (c) women with newly detected hypertension before week 14 did not qualify for enrollment ; (d) labetalol, being considered the drug of choice, was used only by 2/3 of women in the study; (e) systolic BP was ignored when assessing the study outcomes; (f) women with prior severe hypertension were more often randomly allocated to the “less tight” control group. Nevertheless, despite all the above limitations, most experts concluded that the study demonstrated that lowering BP in pregnancy to levels which are routinely achieved by non-pregnant women is safe for the fetus [59]. The investigator-initiated Chronic Hypertension and Pregnancy (CHAP) project included a much larger study group of women with mild chronic hypertension of gestational age less than 23 weeks [60]. A total of 2,408 women were randomized to a BP goal of < 140/90 mmHg (active treatment) or to control treatment, in which antihypertensive medication was withdrawn or never given unless severe hypertension (SBP ≥ 160 mmHg or DBP ≥ 105 mmHg) developed. In the active treatment group, the study participants were supplied with labetalol or extended release nifedipine or other medication such as amlodipine or methyldopa, based on the patient´s preference. The primary outcome was defined as a composite of pre-eclampsia with severe features, medically indicated preterm birth before 35 weeks gestation, placental abruption, or fetal or neonatal death. The primary-outcome events were reduced in the active treatment group (adjusted risk ratio 0.82; 95% CI 0.74–0.92). The overall mean BP was lower in the active treatment group (129.5/79.1 mmHg vs. 132.6/81.5 mmHg). The pre-specified composite maternal or neonatal secondary outcomes did not differ between groups, including small-for-gestational-age newborns. Severe hypertension was less frequent in the active treatment group, with no stroke in either group. The study results support antihypertensive treatment in women with mild pre-existing hypertension to achieve a target BP of < 140/90 mmHg. It should be noted that BP differences were more evident in the first half of the pregnancy. Low doses of aspirin were administered equally in the active treatment and control group (44.6% vs 44.7%).
12 Delivery
Induction of labor is advisable for women with gestational hypertension or mild pre-eclampsia beyond 37 weeks of gestation, as it has been shown to be associated with improved maternal outcome [61]. Factors such as fetal well-being, gestational age, and type of hypertensive disorder determine the optimal timing of delivery. Pre-eclampsia lacking severe features is possibly manageable by expectation. On the other hand, eclampsia requires delivery shortly after the mother is stabilized.
Cesarean delivery should be considered only for obstetric indications and in the rare case of pheochromocytoma. Otherwise, vaginal delivery is preferable for women with hypertension in pregnancy. Severe pre-eclampsia, regardless of gestational age, requires prompt delivery either vaginally or by cesarean section.
During labor and delivery, antihypertensive treatment should continue with the aim of keeping SBP < 160 mmHg and DBP < 90 mmHg.
13 Blood Pressure Postpartum
Fluctuations of blood pressure in the postpartum period are common. After the usual fall in BP following delivery, there is a progressive rise over the subsequent five days. The postpartum period is also associated with a risk of the late onset of pre-eclampsia. Therefore, BP should be checked in all women within six hours of delivery. Transient hypertension may develop postpartum in women who were previously normotensive. This could be due to pain (because of inadequate analgesia), some drugs (non-steroid anti-inflammatory drugs for pain relief, ergot derivatives for postpartum bleeding, or ephedrine), hypervolemia after regional anesthesia, salt and water redistribution into the intravascular compartment, or restoration of non-pregnant vascular tone. Mild hypertension postpartum usually resolves spontaneously. However, as late presentation pre-eclampsia is a possibility, it is necessary to check BP at least once a day for the first five days after delivery. It is advised to continue with BP measuring every other day for at least one week after discharge from hospital.
In the postpartum period up to 4 weeks, hypertensive women with the following symptoms should be suspected of having de novo pre-eclampsia: headaches, epigastric pain (possibly accompanied by nausea and vomiting), visual disturbances (blurred vision, flashing lights, double vision, floating spots, etc., dyspnea (potentially due to pulmonary edema), sudden swelling in the face, hands, or feet, or seizures.
14 Lactation
Generally, breast feeding is not associated with an increase in BP in mothers. Bromocryptin, used to suppress lactation in some countries, may induce hypertension. All antihypertensive drugs are excreted into breast milk, mostly at very low concentrations, except for propranolol, atenolol, acebutolol (potentially inducing signs of neonatal betablockade), and nifedipine, achieving similar levels to those in maternal plasma. According to many guidelines, methyldopa is still considered the drug of choice for breastfeeding mothers, except for women prone to depression.
Labetalol, nifedipine, and enalapril are suggested as first line antihypertensive drugs for breastfeeding mothers by most guidelines. A list of antihypertensive drugs usually compatible with breastfeeding is provided in Table 8. ACE inhibitors can be used in lactating mothers except in cases of premature birth or renal failure in the newborn. Enalapril is the most widely prescribed ACE inhibitor to breastfeeding mothers because of its safety and favorable pharmacokinetics. It is also used for treatment of peripartum cardiomyopathy.
Calcium channel blockers, particularly felodipine and nifedipine, are considered safe and are therefore frequently used, despite nifedipine not being recommended to nursing mothers by the manufacturer. Nifedipine may be the drug of choice in Black women of African or Caribbean origin.
Labetalol and some beta-1 selective blockers, with the most favorable data being available on metoprolol, are also compatible with breastfeeding and are therefore recommended.
Diuretics should be used with caution, as they may reduce milk production.
15 Prognosis After Pregnancy
Women with a history of gestational hypertension or pre-eclampsia are at higher risk of developing hypertension and stroke later in life [62]. Pre-eclampsia was associated with a four-fold increase in heart failure and hypertension, and doubled the risk of ischemic heart disease, stroke, and cardiovascular death [63, 64]. Women with a history of HDP developing hypertension within a decade postpartum were shown to have the most pronounced abnormal echocardiographic findings in left ventricular remodeling and diastolic function, compared to hypertensive women without previous history of HDP [65]. The 2018 American College of Cardiology/American Heart Association cholesterol guidelines suggest initiating treatment with statins in asymptomatic, middle-aged women with an intermediate 10-year risk and history of pre-eclampsia [66]. HDPs are also associated with an increased risk of developing peripartum cardiomyopathy [67, 68].
Women with previous gestational hypertension may develop endothelial dysfunction and early alterations of carbohydrate and lipid metabolism. They may also experience a relative hyperandrogenism which could, together with the metabolic abnormalities, partly explain the increased risk of developing CVD in later life [69].
A recent meta-analysis of 5 cohort studies with a total of more than 180,000 women with HDP and more than 2,300,000 women without HDP showed that the risk of all-cause and vascular dementia was substantially elevated in women with HDP (adjusted HR, 1.38; 95% CI 1.18–1.61, p < 0.01) [70]. There is also growing evidence for an increased risk of alterations in developmental cognition in the offspring [71].
Despite women with HDPs being recognized as high-risk individuals by the 2018 ESC/ESH hypertension guidelines, recommendations on systematic check-ups are still lacking. BP monitoring in the first postpartum months is strongly suggested, possibly by implementing BP self-measurement to be reported to the primary care physician or using e-health technology [72]. Larger validation studies with BP self-measurement are ongoing [73].
Cardiologists and general practitioners should include an obstetric history, regardless of the woman’s age, as part of the cardiovascular risk assessment. Unfortunately, this area is currently lacking data from randomized studies. There is general agreement that women with a history of HDP, particularly when associated with gestational diabetes, metabolic syndrome, or fetal growth restriction, should be closely monitored for the development of CVD. The timing of this recommendation is unclear, but experts suggest setting up an initial review 6–12 weeks postpartum and then at 6–12 months [74]. The examination should include BP evaluation and assessment of other modifiable risk factors [74]. There is a need to develop a CVD risk calculator assessing female-specific risk factors, including hypertensive and metabolic disorders of pregnancy [75].
16 Assisted Reproductive Technology
In high-income countries 2–6% of children are conceived using ART, meaning that, worldwide, at least 8,000,000 children have been born using ART [76]. There is convincing evidence that HDPs are increased in all pregnancies following ART, regardless of the type of treatment [77]. This meta-analysis included 66 longitudinal studies (7,038,029 pregnancies and 203,375 following any ART). These results are confirmed by another meta-analysis showing higher odds of HDP and pre-eclampsia in pregnancies after in vitro fertilization (IVF) or in intracytoplasmic sperm fertilization (ICSI). The risk was particularly high in frozen embryo transfer and oocyte donation pregnancies [29]. Women who conceived via ART in their current pregnancy are advised to take a low dose of aspirin to prevent pre-eclampsia [78].
In 2015 a group of Swiss researchers declared that children conceived by ART have cardiovascular dysfunction and could potentially have increased CV risk later in life [79]. A total of 54 young individuals (mean age 16.5 ± 2.3 years) conceived by ART were compared with 43 spontaneously conceived controls of similar age [80]. Both groups were re-examined 5 years later. Premature vascular aging persisted in ART conceived individuals (impaired flow-mediated dilatation of the brachial artery, increased pulse-wave velocity, and carotid intima-media thickness). They also showed significantly higher SBP and DBP values by ambulatory BP monitoring (ABPM), and 8 of them (15.4%) met the ABPM criteria for hypertension. A significantly lower left ventricular diastolic function was found in another study including children conceived by ART (mean age 12.85 ± 5.8 years) compared to spontaneously conceived controls of roughly the same age [81]. The risk of developing left ventricular diastolic alterations was particularly high in individuals born preterm.
17 Conclusions
HDPs complicate about 10% of pregnancies and are associated with increased risk of morbidity and mortality for the mother, fetus, and the newborn. Diagnosis of hypertension in pregnancy is based on BP values (SBP ≥ 140 mmHg and/or diastolic DBP ≥ 90 mmHg) measured in the office or in hospital, preferably on two separate occasions. Ambulatory BP monitoring should be used to rule out white coat hypertension to avoid unnecessary treatment.
Hypertension in pregnancy should be classified as pre-existing hypertension or gestational hypertension. Pre-eclampsia used to be defined as gestational hypertension with significant proteinuria. In 2018 a new definition of pre-eclampsia was introduced, no longer insisting on the presence of proteinuria, but requiring evidence of other maternal organ dysfunction.
Pre-existing hypertension is associated with a 25% increased risk of developing superimposed pre-eclampsia. A low dose of aspirin should be initiated in these women from week 12 to weeks 36 to 37. The same preventive measure is recommended to all women at high risk of pre-eclampsia, such as having had hypertension during a previous pregnancy, currently having chronic kidney disease, autoimmune disease, or diabetes. A low dose of aspirin should also be given to women at moderate risk of pre-eclampsia.
Calcium supplementation is recommended for the prevention of pre-eclampsia only in women with a low dietary intake of calcium (< 600 mg daily). Vitamin D is also suggested in the prevention of pre-eclampsia.
Women with pre-existing hypertension should continue their salt-restricted diet, otherwise a normal diet without salt restriction is advised. Exercise of low to moderate intensity during pregnancy is effective in reducing the risk of developing gestational diabetes and gestational hypertension. Obese women are advised to avoid a weight gain of more than 6.8 kg.
There is a consensus that SBP ≥ 170 or DBP ≥ 110 mmHg is considered an emergency and hospitalization should follow. The selection of antihypertensive drugs and their route of administration should be determined based on the expected time of delivery. Intravenous labetalol seems to be an almost universal drug of choice. For mild and moderate hypertension, methyldopa, oral labetalol, and calcium antagonists (with the most data available for long-acting nifedipine), are drugs of choice. Based on the CHIPS and CHAP projects, the threshold for initiating drug treatment for hypertension in pregnancy may be reduced to 140/90 mmHg.
Vaginal delivery is preferred for women with hypertension in pregnancy, provided there is no obstetric indication for cesarean delivery. Induction of labor after the 37th week, compared with the expectant approach, is associated with a better prognosis in women with gestational hypertension or mild pre-eclampsia. Pre-eclampsia can also newly develop in the postpartum period and should be suspected if a rise in BP is associated with some symptoms (headache, epigastric pain, visual disturbances, dyspnea, edema in the face, hands, feet, or seizures).
All antihypertensive drugs are excreted into breast milk, most of them at very low concentrations. Labetalol, nifedipine, and enalapril are considered safe and are recommended by most guidelines. However, ACE inhibitors should not be used in cases of premature birth or renal failure of the newborn.
Women with a history of HDPs are at higher risk of developing CVD prematurely in later life.
Data availability
Not applicable.
References
Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–241.
Shen M, Tan H, Zhou S, Smith GN, Walker MC, Wen SW. Trajectory of blood pressure change during pregnancy and the role of pre-gravid blood pressure: a functional data analysis approach. Sci Rep. 2017;7(1):6227.
Stergiou GS, Palatini P, Asmar R, Ioannidis JP, Kollias A, Lacy P, et al. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens. 2019;37(3):459–66.
Davis GK, Roberts LM, Mangos GJ, Brown MA. Comparisons of auscultatory hybrid and automated sphygmomanometers with mercury sphygmomanometry in hypertensive and normotensive pregnant women: parallel validation studies. J Hypertens. 2015;33(3):499–505 (discussion -6).
Mounier-Vehier C, Amar J, Boivin JM, Denolle T, Fauvel JP, Plu-Bureau G, et al. Hypertension and pregnancy: expert consensus statement from the French Society of Hypertension, an affiliate of the French Society of Cardiology. Fundam Clin Pharmacol. 2017;31(1):83–103.
van den Born BH, Lip GYH, Brguljan-Hitij J, Cremer A, Segura J, Morales E, et al. ESC Council on hypertension position document on the management of hypertensive emergencies. Eur Heart J Cardiovasc Pharmacother. 2019;5(1):37–46.
O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–68.
Magee LA, Ramsay G, von Dadelszen P. What is the role of out-of-office BP measurement in hypertensive pregnancy? Hypertens Preg. 2008;27(2):95–101.
Ditisheim A, Wuerzner G, Ponte B, Vial Y, Irion O, Burnier M, et al. Prevalence of hypertensive phenotypes after preeclampsia: a prospective cohort study. Hypertension. 2018;71(1):103–9.
Tucker KL, Mort S, Yu LM, Campbell H, Rivero-Arias O, Wilson HM, et al. Effect of self-monitoring of blood pressure on diagnosis of hypertension during higher-risk pregnancy: the BUMP 1 randomized clinical trial. JAMA. 2022;327(17):1656–65.
Chappell LC, Tucker KL, Galal U, Yu LM, Campbell H, Rivero-Arias O, et al. Effect of self-monitoring of blood pressure on blood pressure control in pregnant individuals with chronic or gestational hypertension: the BUMP 2 Randomized Clinical Trial. JAMA. 2022;327(17):1666–78.
Denolle T, Weber JL, Calvez C, Getin Y, Daniel JC, Lurton O, et al. Diagnosis of white coat hypertension in pregnant women with teletransmitted home blood pressure. Hypertens Preg. 2008;27(3):305–13.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041.
Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-pathophysiology and clinical presentations: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(14):1690–702.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Preg Hypertens. 2018;13:291–310.
Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129(11):1254–61.
Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13–22.
Verlohren S, Brennecke SP, Galindo A, Karumanchi SA, Mirkovic LB, Schlembach D, et al. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Preg Hypertens. 2022;27:42–50.
Huhn EA, Kreienbühl A, Hoffmann I, Schoetzau A, Lange S, de Tejada BM, et al. Diagnostic accuracy of different soluble fms-like tyrosine kinase 1 and placental growth factor cut-off values in the assessment of preterm and term preeclampsia: a gestational age matched case-control study. Front Med (Lausanne). 2018;5:325.
Cerdeira AS, O’Sullivan J, Ohuma EO, James T, Papageorghiou AT, Knight M, et al. Performance of soluble fms-like tyrosine kinase-1-to-placental growth factor ratio of ≥85 for ruling in preeclampsia within 4 weeks. Am J Obstet Gynecol. 2021;224(3):322–3.
Gaccioli F, Sovio U, Gong S, Cook E, Charnock-Jones DS, Smith GCS. Increased placental sFLT1 (Soluble fms-Like Tyrosine Kinase Receptor-1) drives the antiangiogenic profile of maternal serum preceding preeclampsia but not fetal growth restriction. Hypertension. 2023;80(2):325–34.
Rossi GP, Seccia TM, Pessina AC. Clinical use of laboratory tests for the identification of secondary forms of arterial hypertension. Crit Rev Clin Lab Sci. 2007;44(1):1–85.
Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco MC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613–22.
Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens. 2019;37(2):229–52.
Lenders JW. Pheochromocytoma and pregnancy: a deceptive connection. Eur J Endocrinol. 2012;166(2):143–50.
Morton A. Primary aldosteronism and pregnancy. Pregnancy Hypertens. 2015;5(4):259–62.
Landau E, Amar L. Primary aldosteronism and pregnancy. Ann Endocrinol (Paris). 2016;77(2):148–60.
Almasi-Hashiani A, Omani-Samani R, Mohammadi M, Amini P, Navid B, Alizadeh A, et al. Assisted reproductive technology and the risk of preeclampsia: an updated systematic review and meta-analysis. BMC Preg Childb. 2019;19(1):149.
Chih HJ, Elias FTS, Gaudet L, Velez MP. Assisted reproductive technology and hypertensive disorders of pregnancy: systematic review and meta-analyses. BMC Preg Childb. 2021;21(1):449.
Sindre HP, Westvik-Johari K, Spangmose AL, Pinborg A, Romundstad LB, Bergh C, et al. Risk of hypertensive disorders in pregnancy after fresh and frozen embryo transfer in assisted reproduction: a population-based cohort study with within-sibship analysis. Hypertension. 2023;80(2):e6–16.
Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2018;10(10):Cd001059.
Woo J, Giurgescu C, Wagner CL. Evidence of an association between vitamin D deficiency and preterm birth and preeclampsia: a critical review. J Midwif Womens Health. 2019;64(5):613–29.
Fogacci S, Fogacci F, Banach M, Michos ED, Hernandez AV, Lip GYH, et al. Vitamin D supplementation and incident preeclampsia: a systematic review and meta-analysis of randomized clinical trials. Clin Nutr. 2020;39(6):1742–52.
Rossi AC, Mullin PM. Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: a systematic review with meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;158(1):9–16.
Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG. 2009;116(6):780–8.
Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145–54.
Cockburn J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1(8273):647–9.
Redman CW, Beilin LJ, Bonnar J. Treatment of hypertension in pregnancy with methyldopa: blood pressure control and side effects. Br J Obstet Gynaecol. 1977;84(6):419–26.
Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014;2014(2):Cd002252.
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007;207(1):Cd002252.
Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2018;10(10):Cd002252.
Bone JN, Sandhu A, Abalos ED, Khalil A, Singer J, Prasad S, et al. Oral antihypertensives for nonsevere pregnancy hypertension: systematic review, network meta- and trial sequential analyses. Hypertension. 2022;79(3):614–28.
Webster K, Fishburn S, Maresh M, Findlay SC, Chappell LC. Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance. BMJ. 2019;366: l5119.
Physical activity and exercise during pregnancy and the postpartum period: ACOG Committee Opinion Summary, Number 804. Obstet Gynecol. 2020;135(4):991–3.
Di Mascio D, Magro-Malosso ER, Saccone G, Marhefka GD, Berghella V. Exercise during pregnancy in normal-weight women and risk of preterm birth: a systematic review and meta-analysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215(5):561–71.
Barakat R, Pelaez M, Cordero Y, Perales M, Lopez C, Coteron J, et al. Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol. 2016;214(5):649.e1-8.
Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG. 2019;126(8):984–95.
Martínez-Vizcaíno V, Sanabria-Martínez G, Fernández-Rodríguez R, Cavero-Redondo I, Pascual-Morena C, Álvarez-Bueno C, et al. Exercise during pregnancy for preventing gestational diabetes mellitus and hypertensive disorders: an umbrella review of randomised controlled trials and an updated meta-analysis. BJOG. 2023;130(3):264–75.
Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ. 2014;348: g1285.
Maxwell C, Gaudet L, Cassir G, Nowik C, McLeod NL, Jacob C, et al. Guideline no. 391-pregnancy and maternal obesity part 1: pre-conception and prenatal care. J Obstet Gynaecol Can. 2019;41(11):1623–40.
Maxwell C, Gaudet L, Cassir G, Nowik C, McLeod NL, Jacob C, et al. Guideline no. 392-pregnancy and maternal obesity part 2: team planning for delivery and postpartum care. J Obstet Gynaecol Can. 2019;41(11):1660–75.
Magee LA, Cham C, Waterman EJ, Ohlsson A, von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327(7421):955–60.
Antza C, Dimou C, Doundoulakis I, Akrivos E, Stabouli S, Haidich AB, et al. The flipside of hydralazine in pregnancy: a systematic review and meta-analysis. Preg Hypertens. 2020;19:177–86.
Sridharan K, Sequeira RP. Drugs for treating severe hypertension in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(9):1906–16.
Cífková R, Johnson MR, Kahan T, Brguljan J, Williams B, Coca A, et al. Peripartum management of hypertension: a position paper of the ESC Council on Hypertension and the European Society of Hypertension. Eur Heart J Cardiovasc Pharmacother. 2020;6(6):384–93.
Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–90.
Duley L, Gülmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):Cd000025.
Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372(5):407–17.
August P. Lowering diastolic blood pressure in non-proteinuric hypertension in pregnancy is not harmful to the fetus and is associated with reduced frequency of severe maternal hypertension. Evid Based Med. 2015;20(4):141.
Tita AT, Szychowski JM, Boggess K, Dugoff L, Sibai B, Lawrence K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386(19):1781–92.
Koopmans CM, Bijlenga D, Groen H, Vijgen SM, Aarnoudse JG, Bekedam DJ, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374(9694):979–88.
Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845.
Søndergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. 2020;5(12):1390–8.
Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003497.
Countouris ME, Villanueva FS, Berlacher KL, Cavalcante JL, Parks WT, Catov JM. Association of hypertensive disorders of pregnancy with left ventricular remodeling later in life. J Am Coll Cardiol. 2021;77(8):1057–68.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–350.
Hilfiker-Kleiner D, Haghikia A, Nonhoff J, Bauersachs J. Peripartum cardiomyopathy: current management and future perspectives. Eur Heart J. 2015;36(18):1090–7.
Gammill HS, Chettier R, Brewer A, Roberts JM, Shree R, Tsigas E, et al. Cardiomyopathy and preeclampsia. Circulation. 2018;138(21):2359–66.
Paradisi G, Biaggi A, Savone R, Ianniello F, Tomei C, Caforio L, et al. Cardiovascular risk factors in healthy women with previous gestational hypertension. J Clin Endocrinol Metab. 2006;91(4):1233–8.
Schliep KC, McLean H, Yan B, Qeadan F, Theilen LH, de Havenon A, et al. Association between hypertensive disorders of pregnancy and dementia: a systematic review and meta-analysis. Hypertension. 2023;80(2):257–67.
Escudero C, Kupka E, Ibañez B, Sandoval H, Troncoso F, Wikström AK, et al. Brain vascular dysfunction in mothers and their children exposed to preeclampsia. Hypertension. 2023;80(2):242–56.
Heida KY, Bots ML, de Groot CJ, van Dunné FM, Hammoud NM, Hoek A, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: a Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiol. 2016;23(17):1863–79.
Kitt J, Frost A, Mollison J, Tucker KL, Suriano K, Kenworthy Y, et al. Postpartum blood pressure self-management following hypertensive pregnancy: protocol of the Physician Optimised Post-partum Hypertension Treatment (POP-HT) trial. BMJ Open. 2022;12(2): e051180.
Spaan J, Peeters L, Spaanderman M, Brown M. Cardiovascular risk management after a hypertensive disorder of pregnancy. Hypertension. 2012;60(6):1368–73.
Arnott C, Patel S, Hyett J, Jennings G, Woodward M, Celermajer DS. Women and cardiovascular disease: pregnancy, the forgotten risk factor. Heart Lung Circ. 2020;29(5):662–7.
Yang H, Kuhn C, Kolben T, Ma Z, Lin P, Mahner S, et al. Early life oxidative stress and long-lasting cardiovascular effects on offspring conceived by assisted reproductive technologies: a review. Int J Mol Sci. 2020;21(15):5175.
Thomopoulos C, Salamalekis G, Kintis K, Andrianopoulou I, Michalopoulou H, Skalis G, et al. Risk of hypertensive disorders in pregnancy following assisted reproductive technology: overview and meta-analysis. J Clin Hypertens (Greenwich). 2017;19(2):173–83.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43.
Scherrer U, Rexhaj E, Allemann Y, Sartori C, Rimoldi SF. Cardiovascular dysfunction in children conceived by assisted reproductive technologies. Eur Heart J. 2015;36(25):1583–9.
Meister TA, Rimoldi SF, Soria R, von Arx R, Messerli FH, Sartori C, et al. Association of assisted reproductive technologies with arterial hypertension during adolescence. J Am Coll Cardiol. 2018;72(11):1267–74.
Cui L, Zhao M, Zhang Z, Zhou W, Lv J, Hu J, et al. Assessment of cardiovascular health of children ages 6 to 10 years conceived by assisted reproductive technology. JAMA Netw Open. 2021;4(11): e2132602.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access publishing supported by the National Technical Library in Prague.
Conflict of interest
The author declares no financial or non-financial conflicts of interest, directly or indirectly related to the work submitted.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cífková, R. Hypertension in Pregnancy: A Diagnostic and Therapeutic Overview. High Blood Press Cardiovasc Prev 30, 289–303 (2023). https://doi.org/10.1007/s40292-023-00582-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-023-00582-5