Abstract
Background
Primary mucosal melanomas (PMMs) are rare and clinically heterogeneous, including head and neck (HNMs), vulvovaginal (VVMs), conjunctival (CjMs), anorectal (ARMs) and penile (PMs) melanomas. While the prognosis of advanced cutaneous melanoma has noticeably improved using treatments with immune checkpoint inhibitors (ICIs) and molecules targeting BRAF and MEK, few advances have been made for PMMs because of their poorer response to ICIs and their different genetic profile. This prompted us to conduct a systematic review of molecular studies of PMMs to clarify their pathogenesis and potential therapeutic targets.
Methods
All articles that examined gene mutations in PMMs were identified from the databases and selected based on predefined inclusion criteria. Mutation rate was calculated for all PMMs and each location group by relating the number of mutations identified to the total number of samples analysed.
Results
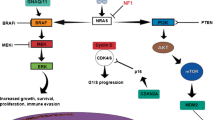
Among 1,581 studies identified, 88 were selected. Overall, the frequency of KIT, BRAF and NRAS mutation was 13.5%, 12.9% and 12.1%, respectively. KIT mutation ranged from 6.4% for CjMs to 16.6% for ARMs, BRAF mutation from 8.6% for ARMs to 31.1% for CjMs, and NRAS mutation from 6.2% for ARMs to 18.5% for CjMs. Among 101 other genes analysed, 33 had mutation rates over 10%, including TTN, TSC1, POM121, NF1, MTOR and SF3B1.
Conclusion
In addition to BRAF, NRAS and KIT genes commonly studied, our systematic review identified significantly mutated genes that have already been associated (e.g., TSC1, mTOR, POLE or ATRX) or could be associated with (future) targeted therapies.
PROSPERO ID: CRD42020185552


Similar content being viewed by others
References
Beaudoux O, Riffaud L, Barbe C, Grange F. Prognostic factors and incidence of primary mucosal melanoma: a population-based study in France. Eur J Dermatol EJD. 2018;28(5):654–60.
Furney SJ, Turajlic S, Stamp G, Nohadani M, Carlisle A, Thomas JM, et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol. 2013;230(3):261–9.
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47.
Mignard C, Deschamps Huvier A, Gillibert A, Duval Modeste AB, Dutriaux C, Khammari A, et al. Efficacy of immunotherapy in patients with metastatic mucosal or uveal melanoma. J Oncol. 2018;2018:1908065.
Mao L, Qi Z, Zhang L, Guo J, Si L. Immunotherapy in acral and mucosal melanoma: current status and future directions. Front Immunol. 2021;12:680407.
Network CGA. Genomic classification of cutaneous melanoma. Cell. 2015;161(7):1681–96.
Noy NF, Shah NH, Whetzel PL, Dai B, Dorf M, Griffith N, et al. BioPortal: ontologies and integrated data resources at the click of a mouse. Nucleic Acids Res. 2009;37:W170-173.
Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47(D1):D941–7.
Liang KV, Sanderson SO, Nowakowski GS, Arora AS. Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin Proc. 2006;81(4):511–6.
Lyle P, Amato C, Fitzpatrick J, Robinson W. Gastrointestinal melanoma or clear cell sarcoma? Molecular evaluation of 7 cases previously diagnosed as malignant melanoma. Am J Surg Pathol. 2008;32:858–66.
Newell F, Kong Y, Wilmott JS, Johansson PA, Ferguson PM, Cui C, et al. Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets. Nat Commun. 2019;10(1):3163.
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80.
Yamashita C, Otsuka A, Nomura M, Honda T, Kabashima K. Successful treatment of metastatic mucosal melanoma with a Del579 c-KIT mutation by imatinib after treatment of anti-PD-1 antibody. J Eur Acad Dermatol Venereol JEADV. 2019;33(3):e92–3.
Murer C, Kränzlin-Stieger P, French LE, Dummer R, Goldinger SM. Successful treatment with imatinib after nilotinib and ipilimumab in a c-kit-mutated advanced melanoma patient: a case report. Melanoma Res. 2017;27(4):396–8.
Komatsu-Fujii T, Nomura M, Otsuka A, Ishida Y, Dai K, Matsumoto S, et al. Response to imatinib in vaginal melanoma with KIT pVal559Gly mutation previously treated with nivolumab, pembrolizumab and ipilimumab. J Dermatol. 2019;46(6):e203–4.
McKean M, Oba J, Ma J, Roth KG, Wang W-L, Macedo MP, et al. Tyrosine kinase inhibitor and immune checkpoint inhibitor responses in KIT-mutant metastatic melanoma. J Invest Dermatol. 2019;139(3):728–31.
Minor DR, Kashani-Sabet M, Garrido M, O’Day SJ, Hamid O, Bastian BC. Sunitinib therapy for melanoma patients with KIT mutations. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18(5):1457–63.
Delyon J, Chevret S, Jouary T, Dalac S, Dalle S, Guillot B, et al. STAT3 mediates nilotinib response in KIT-altered melanoma: a phase II multicenter trial of the french skin cancer network. J Invest Dermatol. 2018;138(1):58–67.
Kalinsky K, Lee S, Rubin KM, Lawrence DP, Iafrarte AJ, Borger DR, et al. A phase 2 trial of dasatinib in patients with locally advanced or stage IV mucosal, acral, or vulvovaginal melanoma: A trial of the ECOG-ACRIN Cancer Research Group (E2607). Cancer. 2017;123(14):2688–97.
Guo J, Si L, Kong Y, Flaherty KT, Xu X, Zhu Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(21):2904–9.
Lee SJ, Kim TM, Kim YJ, Jang K-T, Lee HJ, Lee SN, et al. Phase II trial of nilotinib in patients with metastatic malignant melanoma harboring KIT gene aberration: a multicenter trial of Korean cancer study group (UN10-06). Oncologist. 2015;20(11):1312–9.
Carvajal RD, Spencer SA, Lydiatt W. Mucosal melanoma: a clinically and biologically unique disease entity. J Natl Compr Cancer Netw JNCCN. 2012;10(3):345–56.
Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs. 2012;30(5):2008–14.
Wang K, Yamamoto H, Chin JR, Werb Z, Vu TH. Epidermal growth factor receptor-deficient mice have delayed primary endochondral ossification because of defective osteoclast recruitment. J Biol Chem. 2004;279(51):53848–56.
Simiczyjew A, Pietraszek-Gremplewicz K, Dratkiewicz E, Podgórska M, Matkowski R, Ziętek M, et al. Combination of selected MET and EGFR inhibitors decreases melanoma cells’ invasive abilities. Front Pharmacol. 2019;10:1116.
Dumaz N, Jouenne F, Delyon J, Mourah S, Bensussan A, Lebbé C. Atypical BRAF and NRAS mutations in mucosal melanoma. Cancers. 2019;11:8.
Gajewski TF, Salama AKS, Niedzwiecki D, Johnson J, Linette G, Bucher C, et al. Phase II study of the farnesyltransferase inhibitor R115777 in advanced melanoma (CALGB 500104). J Transl Med. 2012;10:246.
Dummer R, Schadendorf D, Ascierto PA, Arance A, Dutriaux C, Di Giacomo AM, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(4):435–45.
Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37(24):3183–99.
Bai X, Mao LL, Chi ZH, Sheng XN, Cui CL, Kong Y, et al. BRAF inhibitors: efficacious and tolerable in BRAF-mutant acral and mucosal melanoma. Neoplasma. 2017;64(4):626–32.
Zhu J, Li C, Yang H, Guo X, Huang T, Han W. Computational study on the effect of inactivating/activating mutations on the inhibition of MEK1 by trametinib. Int J Mol Sci. 2020;21:6.
Arafeh R, Di Pizio A, Elkahloun AG, Dym O, Niv MY, Samuels Y. RASA2 and NF1; two-negative regulators of Ras with complementary functions in melanoma. Oncogene. 2019;38(13):2432–4.
Bakhoum MF, Esmaeli B. Molecular Characteristics of Uveal Melanoma: Insights from the Cancer Genome Atlas (TCGA) Project. Cancers [Internet]. 27 juill 2019 [cité 10 avr 2020];11(8). Disponible sur: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6721321/
Chua V, Aplin AE. Novel therapeutic strategies and targets in advanced uveal melanoma. Curr Opin Oncol. 2018;30(2):134–41.
Kong Y, Si L, Li Y, Wu X, Xu X, Dai J, et al. Analysis of mTOR gene aberrations in melanoma patients and evaluation of their sensitivity to PI3K-AKT-mTOR pathway inhibitors. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22(4):1018–27.
Yu J, Wu X, Yan J, Yu J, Yin T, Dai J, et al. Potential mutations in uveal melanoma identified using targeted next-generation sequencing. J Cancer. 2019;10(2):488–93.
Amirouchene-Angelozzi N, Frisch-Dit-Leitz E, Carita G, Dahmani A, Raymondie C, Liot G, et al. The mTOR inhibitor Everolimus synergizes with the PI3K inhibitor GDC0941 to enhance anti-tumor efficacy in uveal melanoma. Oncotarget. 2016;7(17):23633–46.
McWilliams RR, Allred JB, Slostad JA, Katipamula R, Dronca RS, Rumilla KM, et al. NCCTG N0879 (Alliance): a randomized phase 2 cooperative group trial of carboplatin, paclitaxel, and bevacizumab ± everolimus for metastatic melanoma. Cancer. 2018;124(3):537–45.
Dronca RS, Allred JB, Perez DG, Nevala WK, Lieser EAT, Thompson M, et al. Phase II study of temozolomide (TMZ) and everolimus (RAD001) therapy for metastatic melanoma: a North Central Cancer Treatment Group study, N0675. Am J Clin Oncol. 2014;37(4):369–76.
Shoushtari AN, Ong LT, Schoder H, Singh-Kandah S, Abbate KT, Postow MA, et al. A phase 2 trial of everolimus and pasireotide long-acting release in patients with metastatic uveal melanoma. Melanoma Res. 2016;26(3):272–7.
Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552–61.
Bargonetti J, Prives C. Gain-of-function mutant p53: history and speculation. J Mol Cell Biol. 2019;11(7):605–9.
Klein JD, Kupferman ME. Li-Fraumeni syndrome presenting as mucosal melanoma: case report and treatment considerations. Head Neck. 2017;39(2):E20–2.
Fritsche MK, Knopf A. The tumor suppressor p53 in mucosal melanoma of the head and neck. Genes. 2017;8:12.
Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102.
Pritchard AL, Johansson PA, Nathan V, Howlie M, Symmons J, Palmer JM, et al. Germline mutations in candidate predisposition genes in individuals with cutaneous melanoma and at least two independent additional primary cancers. PLoS ONE. 2018;13(4):e0194098.
Aoude LG, Heitzer E, Johansson P, Gartside M, Wadt K, Pritchard AL, et al. POLE mutations in families predisposed to cutaneous melanoma. Fam Cancer déc. 2015;14(4):621–8.
Wang F, Zhao Q, Wang Y-N, Jin Y, He M-M, Liu Z-X, et al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol. 2019;5(10):1504–6.
Toussi A, Mans N, Welborn J, Kiuru M. Germline mutations predisposing to melanoma. J Cutan Pathol. 2020;2:2.
Lee B, McArthur GA. CDK4 inhibitors an emerging strategy for the treatment of melanoma. Melanoma Manag. 2015;2(3):255–66.
Delyon J, Lebbe C, Dumaz N. Targeted therapies in melanoma beyond BRAF: targeting NRAS-mutated and KIT-mutated melanoma. Curr Opin Oncol. 2020;32(2):79–84.
Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704.
Colebatch AJ, Dobrovic A, Cooper WA. TERT gene: its function and dysregulation in cancer. J Clin Pathol. 2019;72(4):281–4.
Yuan X, Larsson C, Xu D. Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players. Oncogene. 2019;38(34):6172–83.
Chiba K, Lorbeer FK, Shain AH, McSwiggen DT, Schruf E, Oh A, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science. 2017;357(6358):1416–20.
Vallarelli AF, Rachakonda PS, André J, Heidenreich B, Riffaud L, Bensussan A, et al. TERT promoter mutations in melanoma render TERT expression dependent on MAPK pathway activation. Oncotarget. 2016;7(33):53127–36.
Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–61.
Motaparthi K, Kim J, Andea AA, Missall TA, Novoa RA, Vidal CI, et al. TERT and TERT promoter in melanocytic neoplasms: Current concepts in pathogenesis, diagnosis, and prognosis. J Cutan Pathol. 2020;47(8):710–9.
Ren X, Tu C, Tang Z, Ma R, Li Z. Alternative lengthening of telomeres phenotype and loss of ATRX expression in sarcomas. Oncol Lett. 2018;15(5):7489–96.
Iles MM, Bishop DT, Taylor JC, Hayward NK, Brossard M, Cust AE, et al. The Effect on Melanoma Risk of Genes Previously Associated With Telomere Length. JNCI J Natl Cancer Inst [Internet]. 2014 [cité 6 mai 2020];106(10). Disponible sur: https://academic.oup.com/jnci/article/106/10/dju267/2965093
Qadeer ZA, Harcharik S, Valle-Garcia D, Chen C, Birge MB, Vardabasso C, et al. Decreased expression of the chromatin remodeler ATRX associates with melanoma progression. J Invest Dermatol. 2014;134(6):1768–72.
Liang J, Zhao H, Diplas BH, Liu S, Liu J, Wang D, et al. Genome-wide CRISPR-Cas9 screen reveals selective vulnerability of ATRX-mutant cancers to WEE1 inhibition. Cancer Res. 2019;2:2.
Cole KA, Pal S, Kudgus RA, Ijaz H, Liu X, Minard CG, et al. Phase I clinical trial of the Wee1 inhibitor adavosertib (AZD1775) with irinotecan in children with relapsed solid tumors: a COG phase I consortium report (ADVL1312). Clin Cancer Res. 2020;26(6):1213–9.
Geenen JJJ, Schellens JHM. Molecular pathways: targeting the protein kinase Wee1 in cancer. Clin Cancer Res. 2017;23(16):4540–4.
Quek C, Rawson RV, Ferguson PM, Shang P, Silva I, Saw RPM, et al. Recurrent hotspot SF3B1 mutations at codon 625 in vulvovaginal mucosal melanoma identified in a study of 27 Australian mucosal melanomas. Oncotarget. 2019;10(9):930–41.
Seiler M, Yoshimi A, Darman R, Chan B, Keaney G, Thomas M, et al. H3B–8800, an orally available small-molecule splicing modulator, induces lethality in spliceosome-mutant cancers. Nat Med mai. 2018;24(4):497–504.
Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–72.
Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716–28.
Aktary Z, Bertrand JU, Larue L. The WNT-less wonder: WNT-independent β-catenin signaling. Pigment Cell Melanoma Res. 2016;29(5):524–40.
Lorusso P, Chawla SP, Bendell J, Shields AF, Shapiro G, Rajagopalan P, et al. First-in-human study of the monopolar spindle 1 (Mps1) kinase inhibitor BAY 1161909 in combination with paclitaxel in subjects with advanced malignancies. Ann Oncol. 2018;29:138.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received.
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval statement
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consent for publication.
Data availability statement
Data that support the findings of this study are available on request from the corresponding author.
Code availability
The algorithm is available in the manuscript.
Author contributions
O. Beaudoux and F. Grange conceived of the presented idea and contributed to the design and implementation of the research. O. Beaudoux developed the theory and performed the analysis of the results. F. Grange and A.S. Lebre verified the analytical methods and supervised the findings of this work. All authors discussed the results, participated in the writing of the manuscript, and contributed to the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beaudoux, O., Oudart, JB., Riffaud, L. et al. Mutational Characteristics of Primary Mucosal Melanoma: A Systematic Review. Mol Diagn Ther 26, 189–202 (2022). https://doi.org/10.1007/s40291-021-00572-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-021-00572-0