Abstract
Background
Quality of life (QoL) is an important outcome to capture in clinical trials evaluating deprescribing interventions.
Objective
We aimed to conduct a scoping review to examine how QoL has been measured in deprescribing trials among older people and identify potentially relevant QoL scales, to better inform QoL measurement in future deprescribing trials.
Methods
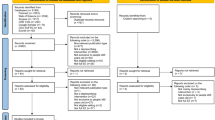
We searched MEDLINE, Embase, PsycINFO, the Cochrane Central Register of Controlled Trials, Google Scholar, Epistemonikos, ClinicalTrials.gov, and reference lists of eligible studies (from inception to October 2023). We included randomized and non-randomized comparative studies with a control group that evaluated deprescribing and polypharmacy reduction interventions in people ≥ 65 years of age and measured QoL as an outcome. We also included studies describing the development and validation of QoL scales related to deprescribing, polypharmacy, or medication burden in adults ≥ 18 years of age. Two independent reviewers screened titles and abstracts, then full texts. Two independent reviewers extracted data from 25% of eligible studies in order to verify agreement, then a single reviewer extracted data from the remaining studies, which a second reviewer cross-checked. We critically appraised scales based on the COSMIN checklist.
Results
We retrieved 7290 articles, of which 52 were eligible for inclusion, including 44 deprescribing trials and eight scale development studies. From these studies, we found 21 scales that have been used in the context of deprescribing/polypharmacy (12 generic scales used in clinical trials and nine medication-specific scales). Variations of the generic EQ-5D were the most used scales. The measurement properties of scales for capturing changes in QoL from deprescribing were uncertain. Medication-specific QoL scales have not been employed in deprescribing clinical trials and thus, their performance in this context is also not clear.
Conclusions
Several existing QoL scales have been applied to the context of deprescribing/polypharmacy clinical trials, and new scales specific to the problem have been proposed. If deprescribing does impact QoL, our findings suggest it is uncertain whether existing QoL scales can practically and reliably capture such a change or whether any scale is best. However, this review compares various aspects of the scales that researchers and clinicians can consider in decisions about measuring QoL in deprescribing trials, and in planning future research.
Protocol Registration
Open Science Framework: osf.io/aez6w.



Similar content being viewed by others
References
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):1–10. https://doi.org/10.1186/s12877-017-0621-2.
Wastesson JW, Morin L, Tan ECK, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17(12):1185–96. https://doi.org/10.1080/14740338.2018.1546841.
Woodward MC. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33(4):323–8. https://doi.org/10.1002/jppr2003334323.
Reeve E, Gnjidic D, Long J, Hilmer S. A systematic review of the emerging definition of “deprescribing” with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol. 2015;80(6):1254–68. https://doi.org/10.1111/bcp.12732.
Beuscart JB, Knol W, Cullinan S, et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018;16(1):21. https://doi.org/10.1186/s12916-018-1007-9.
Rankin A, Cadogan CA, Ryan C, Clyne B, Smith SM, Hughes CM. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc. 2018. https://doi.org/10.1111/jgs.15245.
Aubert CE, Kerr EA, Maratt JK, Klamerus ML, Hofer TP. Outcome measures for interventions to reduce inappropriate chronic drugs: a narrative review. J Am Geriatr Soc. 2020;68(10):2390–8. https://doi.org/10.1111/jgs.16697.
Pruskowski JA, Springer S, Thorpe CT, Klein-Fedyshin M, Handler SM. Does deprescribing improve quality of life? A systematic review of the literature. Drugs Aging. 2019;36(12):1097–110. https://doi.org/10.1007/s40266-019-00717-1.
McDonald EG, Wu PE, Rashidi B, et al. The MedSafer study: electronic decision support for deprescribing in hospitalized older adults. JAMA Intern Med. 2022;182(3):265. https://doi.org/10.1001/jamainternmed.2021.7429.
Blum MR, Sallevelt BTGM, Spinewine A, et al. Optimizing therapy to prevent avoidable hospital admissions in multimorbid older adults (OPERAM): cluster randomised controlled trial. BMJ. 2021;374: n1585. https://doi.org/10.1136/bmj.n1585.
Ibrahim K, Cox NJ, Stevenson JM, Lim S, Fraser SDS, Roberts HC. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr. 2021;21(1):258. https://doi.org/10.1186/s12877-021-02208-8.
Lundby C, Pottegård A. Considerations regarding choice of primary outcome in clinical trials in deprescribing. Br J Clin Pharmacol. 2022;88(7):3032–4. https://doi.org/10.1111/bcp.14990.
James KA, Cadel L, Hitzig SL, Guilcher SJT. Patient-reported outcome measures for medication-related quality of life: a scoping review. Res Soc Admin Pharm. 2022;18(9):3501–23. https://doi.org/10.1016/j.sapharm.2022.03.003.
Jennings ELM, O’Mahony D, Gallagher PF. Medication-related quality of life (MRQoL) in ambulatory older adults with multi-morbidity and polypharmacy. Eur Geriatr Med. 2022;13(3):579–83. https://doi.org/10.1007/s41999-021-00573-6.
Bhadhuri A, Kind P, Salari P, et al. Measurement properties of EQ-5D-3L and EQ-5D-5L in recording self-reported health status in older patients with substantial multimorbidity and polypharmacy. Health Qual Life Outcomes. 2020;18(1):317. https://doi.org/10.1186/s12955-020-01564-0.
Holmes HM, Hayley DC, Alexander GC, Sachs GA. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166(6):605–9. https://doi.org/10.1001/archinte.166.6.605.
Hilmer SN, McLachlan AJ, Le Couteur DG. Clinical pharmacology in the geriatric patient. Fundam Clin Pharmacol. 2007;21(3):217–30. https://doi.org/10.1111/j.1472-8206.2007.00473.x.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. https://doi.org/10.1186/s12874-018-0611-x.
Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13(1):48. https://doi.org/10.1186/1471-2288-13-48.
Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5(4):371–85. https://doi.org/10.1002/jrsm.1123.
Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850.
JBI. JBI manual for evidence synthesis. 2022. https://jbi-global-wiki.refined.site/space/MANUAL. Accessed 27 Sep 2022.
Thompson W, Xi C, Hughes C, et al. Measuring quality of life in deprescribing trials: a scoping review protocol. Open Science Framework. 2022. https://osf.io/aez6w. Accessed 10 Sep 2023.
Gnjidic D, Reeve E. Deprescribing: what do we know, and where to next? Br J Clin Pharmacol. 2021;87(3):722–4. https://doi.org/10.1111/bcp.14525.
Mohammed MA, Moles RJ, Hilmer SN, Kouladjian O’Donnel L, Chen TF. Development and validation of an instrument for measuring the burden of medicine on functioning and well-being: the Medication-Related Burden Quality of Life (MRB-QoL) tool. BMJ Open. 2018;8(1): e018880. https://doi.org/10.1136/bmjopen-2017-018880.
Harzing AW. Publish or perish. 2007. https://harzing.com/resources/publish-or-perish. Accessed 1 Apr 2023.
Mokkink LB, Terwee CB, Knol DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol. 2010;10(1):22. https://doi.org/10.1186/1471-2288-10-22.
Siette J, Knaggs GT, Zurynski Y, Ratcliffe J, Dodds L, Westbrook J. Systematic review of 29 self-report instruments for assessing quality of life in older adults receiving aged care services. BMJ Open. 2021;11(11): e050892. https://doi.org/10.1136/bmjopen-2021-050892.
Anderson K, Freeman C, Foster M, Scott I. GP-Led Deprescribing in community-living older Australians: an exploratory controlled trial. J Am Geriatr Soc. 2020;68(2):403–10. https://doi.org/10.1111/jgs.16273.
Ashizawa T, Mishina S, Igarashi A, Kobayashi T, Takase Y, Ikeda S. Improvement in prescriptions while maintaining overall health outcomes: a prospective observational study conducted in Japanese facilities for older people. BMC Geriatr. 2022;22(1):323. https://doi.org/10.1186/s12877-022-02872-4.
Balsom C, Pittman N, King R, Kelly D. Impact of a pharmacist-administered deprescribing intervention on nursing home residents: a randomized controlled trial. Int J Clin Pharm. 2020;42(4):1153–67. https://doi.org/10.1007/s11096-020-01073-6.
Beer C, Loh P, Peng YG, Potter K, Millar A. A pilot randomized controlled trial of deprescribing. Ther Adv Drug Saf. 2011;2(2):37–43. https://doi.org/10.1177/2042098611400332.
Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Qual Saf. 2011;20(9):738–46. https://doi.org/10.1136/bmjqs.2009.039693.
Bryant LJM, Coster G, Gamble GD, McCormick RN. The General Practitioner-Pharmacist Collaboration (GPPC) study: a randomised controlled trial of clinical medication reviews in community pharmacy. Int J Pharm Pract. 2011;19(2):94–105. https://doi.org/10.1111/j.2042-7174.2010.00079.x.
Callegari E, Benth JŠ, Selbæk G, Grønnerød C, Bergh S. The effect of the NorGeP–NH on quality of life and drug prescriptions in Norwegian nursing homes: a randomized controlled trial. Pharmacy. 2022;10(1):32. https://doi.org/10.3390/pharmacy10010032.
Campins L, Serra-Prat M, Gózalo I, et al. Randomized controlled trial of an intervention to improve drug appropriateness in community-dwelling polymedicated elderly people. Fam Pract. 2017;34(1):36–42. https://doi.org/10.1093/fampra/cmw073.
Cateau D, Ballabeni P, Niquille A. Effects of an interprofessional deprescribing intervention in Swiss nursing homes: the Individual Deprescribing Intervention (IDeI) randomised controlled trial. BMC Geriatr. 2021;21(1):655. https://doi.org/10.1186/s12877-021-02465-7.
Curtin D, Jennings E, Daunt R, et al. Deprescribing in older people approaching end of life: a randomized controlled trial using STOPPFrail criteria. J Am Geriatr Soc. 2020;68(4):762–9. https://doi.org/10.1111/jgs.16278.
del Cura-González I, López-Rodríguez JA, Leiva-Fernández F, et al. How to improve healthcare for patients with multimorbidity and polypharmacy in primary care: a pragmatic cluster-randomized clinical trial of the MULTIPAP intervention. J Pers Med. 2022;12(5):752. https://doi.org/10.3390/jpm12050752.
Etherton-Beer C, Page A, Naganathan V, et al. Deprescribing to optimise health outcomes for frail older people: a double-blind placebo-controlled randomised controlled trial: outcomes of the Opti-med study. Age Ageing. 2023;52(5):afad081. https://doi.org/10.1093/ageing/afad081.
Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc. 2014;62(9):1658–65. https://doi.org/10.1111/jgs.12993.
Gillespie P, Clyne B, Raymakers A, Fahey T, Hughes CM, Smith SM. Reducing potentially inappropriate prescribing for older people in primary care: cost-effectiveness of the OPTI-SCRIPT intervention. Int J Technol Assess Health Care. 2017;33(4):494–503. https://doi.org/10.1017/S0266462317000782.
Grischott T, Rachamin Y, Senn O, Hug P, Rosemann T, Neuner-Jehle S. Medication review and enhanced information transfer at discharge of older patients with polypharmacy: a cluster-randomized controlled trial in Swiss hospitals. J Gen Intern Med. 2023;38(3):610–8. https://doi.org/10.1007/s11606-022-07728-6.
Jódar-Sánchez F, Malet-Larrea A, Martín JJ, et al. Cost-utility analysis of a medication review with follow-up service for older adults with polypharmacy in community pharmacies in Spain: the conSIGUE program. Pharmacoeconomics. 2015;33(6):599–610. https://doi.org/10.1007/s40273-015-0270-2.
Kornholt J, Feizi ST, Hansen AS, et al. Effects of a comprehensive medication review intervention on health-related quality of life and other clinical outcomes in geriatric outpatients with polypharmacy: a pragmatic randomized clinical trial. Br J Clin Pharmacol. 2022;88(7):3360–9. https://doi.org/10.1111/bcp.15287.
Lenaghan E, Holland R, Brooks A. Home-based medication review in a high risk elderly population in primary care: the POLYMED randomised controlled trial. Age Ageing. 2007;36(3):292–7. https://doi.org/10.1093/ageing/afm036.
Lin HW, Lin CH, Chang CK, et al. Economic outcomes of pharmacist-physician medication therapy management for polypharmacy elderly: a prospective, randomized, controlled trial. J Formos Med Assoc. 2018;117(3):235–43. https://doi.org/10.1016/j.jfma.2017.04.017.
Liou WS, Huang SM, Lee WH, Chang YL, Wu MF. The effects of a pharmacist-led medication review in a nursing home. Medicine. 2021;100(48): e28023. https://doi.org/10.1097/MD.0000000000028023.
Mahlknecht A, Wiedermann CJ, Sandri M, et al. Expert-based medication reviews to reduce polypharmacy in older patients in primary care: a northern-Italian cluster-randomised controlled trial. BMC Geriatr. 2021;21(1):659. https://doi.org/10.1186/s12877-021-02612-0.
Mangin D, Lamarche L, Agarwal G, et al. Team approach to polypharmacy evaluation and reduction: feasibility randomized trial of a structured clinical pathway to reduce polypharmacy. Pilot Feasibility Stud. 2023;9(1):84. https://doi.org/10.1186/s40814-023-01315-0.
McCarthy C, Clyne B, Boland F, et al. GP-delivered medication review of polypharmacy, deprescribing, and patient priorities in older people with multimorbidity in Irish primary care (SPPiRE Study): a cluster randomised controlled trial. PLoS Med. 2022;19(1): e1003862. https://doi.org/10.1371/journal.pmed.1003862.
Moga DC, Abner EL, Rigsby DN, et al. Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden. Alzheimers Res Ther. 2017;9(1):36. https://doi.org/10.1186/s13195-017-0263-9.
Muth C, Harder S, Uhlmann L, et al. Pilot study to test the feasibility of a trial design and complex intervention on PRI oritising MU ltimedication in M ultimorbidity in general practices (PRIMUM pilot). BMJ Open. 2016;6(7): e011613. https://doi.org/10.1136/bmjopen-2016-011613.
Muth C, Uhlmann L, Haefeli WE, et al. Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open. 2018;8(2): e017740. https://doi.org/10.1136/bmjopen-2017-017740.
Olsson IN, Runnamo R, Engfeldt P. Drug treatment in the elderly: an intervention in primary care to enhance prescription quality and quality of life. Scand J Prim Health Care. 2012;30(1):3–9. https://doi.org/10.3109/02813432.2011.629149.
O’Mahony D, Gudmundsson A, Soiza RL, et al. Prevention of adverse drug reactions in hospitalized older patients with multi-morbidity and polypharmacy: the SENATOR* randomized controlled clinical trial. Age Ageing. 2020;49(4):605–14. https://doi.org/10.1093/ageing/afaa072.
Pitkälä KH, Juola AL, Kautiainen H, et al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. J Am Med Dir Assoc. 2014;15(12):892–8. https://doi.org/10.1016/j.jamda.2014.04.002.
Polinder S, Boyé NDA, Mattace-Raso FUS, et al. Cost-utility of medication withdrawal in older fallers: results from the improving medication prescribing to reduce risk of FALLs (IMPROveFALL) trial. BMC Geriatr. 2016;16(1):179. https://doi.org/10.1186/s12877-016-0354-7.
Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLoS ONE. 2016;11(3): e0149984. https://doi.org/10.1371/journal.pone.0149984.
Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369: m1822. https://doi.org/10.1136/bmj.m1822.
Romskaug R, Skovlund E, Straand J, et al. Effect of clinical geriatric assessments and collaborative medication reviews by geriatrician and family physician for improving health-related quality of life in home-dwelling older patients receiving polypharmacy. JAMA Intern Med. 2020;180(2):181. https://doi.org/10.1001/jamainternmed.2019.5096.
Roughead EE, Pratt NL, Parfitt G, et al. Effect of an ongoing pharmacist service to reduce medicine-induced deterioration and adverse reactions in aged-care facilities (nursing homes): a multicentre, randomised controlled trial (the ReMInDAR trial). Age Ageing. 2022;51(4):afac092. https://doi.org/10.1093/ageing/afac092.
Sakakibara M, Igarashi A, Takase Y, Kamei H, Nabeshima T. Effects of prescription drug reduction on quality of life in community-dwelling patients with dementia. J Pharm Pharm Sci. 2015;18(5):705. https://doi.org/10.18433/J37P5X.
Syafhan NF, Al Azzam S, Williams SD, et al. General practitioner practice-based pharmacist input to medicines optimisation in the UK: pragmatic, multicenter, randomised, controlled trial. J Pharm Policy Pract. 2021;14(1):4. https://doi.org/10.1186/s40545-020-00279-3.
Van der Linden L, Decoutere L, Walgraeve K, et al. Combined use of the Rationalization of home medication by an Adjusted STOPP in older Patients (RASP) list and a pharmacist-led medication review in very old inpatients: impact on quality of prescribing and clinical outcome. Drugs Aging. 2017;34(2):123–33. https://doi.org/10.1007/s40266-016-0424-8.
van der Meer HG, Wouters H, Pont LG, Taxis K. Reducing the anticholinergic and sedative load in older patients on polypharmacy by pharmacist-led medication review: a randomised controlled trial. BMJ Open. 2018;8(7): e019042. https://doi.org/10.1136/bmjopen-2017-019042.
Verdoorn S, Kwint HF, Blom JW, Gussekloo J, Bouvy ML. Effects of a clinical medication review focused on personal goals, quality of life, and health problems in older persons with polypharmacy: a randomised controlled trial (DREAMeR-study). PLoS Med. 2019;16(5): e1002798. https://doi.org/10.1371/journal.pmed.1002798.
Williams ME, Pulliam CC, Hunter R, et al. The short-term effect of interdisciplinary medication review on function and cost in ambulatory elderly people. J Am Geriatr Soc. 2004;52(1):93–8. https://doi.org/10.1111/j.1532-5415.2004.52016.x.
Wouters H, Scheper J, Koning H, et al. Discontinuing inappropriate medication use in nursing home residents. Ann Intern Med. 2017;167(9):609. https://doi.org/10.7326/M16-2729.
Zechmann S, Senn O, Valeri F, et al. Effect of a patient-centred deprescribing procedure in older multimorbid patients in Swiss primary care: a cluster-randomised clinical trial. BMC Geriatr. 2020;20(1):471. https://doi.org/10.1186/s12877-020-01870-8.
Duncan P, Murphy M, Man MS, Chaplin K, Gaunt D, Salisbury C. Development and validation of the Multimorbidity Treatment Burden Questionnaire (MTBQ). BMJ Open. 2020;8(4): e019413. https://doi.org/10.1136/bmjopen-2017-019413.
Eton DT, Yost KJ, Lai J, et al. Development and validation of the Patient Experience with Treatment and Self-management (PETS): a patient-reported measure of treatment burden. Qual Life Res. 2017;26(2):489–503. https://doi.org/10.1007/s11136-016-1397-0.
Eton DT, Linzer M, Boehm DH, et al. Deriving and validating a brief measure of treatment burden to assess person-centered healthcare quality in primary care: a multi-method study. BMC Fam Pract. 2020;21(1):221. https://doi.org/10.1186/s12875-020-01291-x.
Katusiime B, Corlett S, Krska J. Development and validation of a revised instrument to measure burden of long-term medicines use: the Living with Medicines Questionnaire version 3. Patient Relat Outcome Meas. 2018;9:155–8. https://doi.org/10.2147/PROM.S151143.
Sakthong P, Chinthammit C, Sukarnjanaset P, Sonsa-ardjit N, Munpan W. Psychometric properties of the Patient-Reported Outcomes Measure of Pharmaceutical Therapy for Quality of Life (PROMPT-QOL). Value Health Reg Issues. 2017;12:41–9. https://doi.org/10.1016/j.vhri.2017.02.003.
Tran VT, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med. 2014;12(1):109. https://doi.org/10.1186/1741-7015-12-109.
Tseng HM, Lee CH, Chen YJ, Hsu HH, Huang LY, Huang JL. Developing a measure of medication-related quality of life for people with polypharmacy. Qual Life Res. 2016;25(5):1295–302. https://doi.org/10.1007/s11136-015-1177-2.
Gottschalk S, König HH, Nejad M, Dams J. Measurement properties of the EQ-5D in populations with a mean age of ≥ 75 years: a systematic review. Qual Life Res. 2023;32(2):307–29. https://doi.org/10.1007/s11136-022-03185-0.
Schenker Y, Park SY, Jeong K, et al. Associations between polypharmacy, symptom burden, and quality of life in patients with advanced, life-limiting illness. J Gen Intern Med. 2019;34(4):559–66. https://doi.org/10.1007/s11606-019-04837-7.
Aljeaidi MS, Haaksma ML, Tan ECK. Polypharmacy and trajectories of health-related quality of life in older adults: an Australian cohort study. Qual Life Res. 2022;31(9):2663–71. https://doi.org/10.1007/s11136-022-03136-9.
Weir K, Nickel B, Naganathan V, et al. Decision-making preferences and deprescribing: perspectives of older adults and companions about their medicines. J Gerontol B Psychol Sci Soc Sci. 2018;73(7):e98-107. https://doi.org/10.1093/geronb/gbx138.
Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793–807. https://doi.org/10.1007/s40266-013-0106-8.
Farrell B, Merkley VF, Thompson W. Managing polypharmacy in a 77-year-old woman with multiple prescribers. CMAJ. 2013. https://doi.org/10.1503/cmaj.122012.
Sanghera S, Walther A, Peters TJ, Coast J. Challenges in using recommended quality of life measures to assess fluctuating health: a think-aloud study to understand how recall and timing of assessment influence patient responses. Patient. 2022;15(4):445–57. https://doi.org/10.1007/s40271-021-00555-7.
Hakamies-Blomqvist L, Luoma ML, Sjostrom J, et al. Timing of quality of life (QoL) assessments as a source of error in oncological trials. J Adv Nurs. 2001;35(5):709–16. https://doi.org/10.1046/j.1365-2648.2001.01903.x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project is supported by funding from the United States Deprescribing Research Network (National Institute on Aging, Grant No. R24AG064025).
Conflict of interest
Wade Thompson has received grants from the US Deprescribing Network (National Institute on Aging), Canadian Institutes of Health Research, and Health Canada, for research on deprescribing. He also receives salary support from Michael Smith Health Research British Columbia. Wade Thompson, Carina Lundby, and Frank Moriarty were members of the Junior Investigator Intensive Program of the US Deprescribing Research Network, which is funded by the National Institute on Aging (R24AG064025). Jung Ah Hong contributed to this research while on placement supported by funds from the School of Pharmacy and Biomolecular Sciences, RCSI University of Medicine and Health Sciences. Adam Bleik, Harman Waring, Chris Xi, Carmel Hughes, Douglas M. Salzwedel, Emily G. McDonald, Jennifer Pruskowski, Sion Scott, Anne Spinewine, Jean S. Kutner, Trine Graabæk, and Shahrzad Elmi have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data are available on request from the authors.
Code availability
Not applicable.
Author contributions
All authors meet the ICMJE authorship criteria and approve submission. The study was conceived by CL, WT, JP, SS, and FM. All authors contributed to the design of the study. WT, AB, CL, HW, JAH, CX, SE, and FM collected the data. All authors interpreted results. All authors contributed to drafting and critical revisions of the manuscript and approved the final submission.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thompson, W., Lundby, C., Bleik, A. et al. Measuring Quality of Life in Deprescribing Trials: A Scoping Review. Drugs Aging 41, 379–397 (2024). https://doi.org/10.1007/s40266-024-01113-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-024-01113-0