Abstract
Introduction
Worldwide, polypharmacy and medication appropriateness-related outcomes (MARO) are growing public health concerns associated with potentially inappropriate prescribing, adverse health effects, and avoidable costs to health systems. Continuity of care (COC) is a cornerstone of high-quality care that has been shown to improve patient-relevant outcomes. However, the relationship between COC and polypharmacy/MARO has not been systematically explored.
Objective
The aim of this systematic review was to investigate the operationalization of COC, polypharmacy, and MARO as well as the relationship between COC and polypharmacy/MARO.
Methods
We performed a systematic literature search in PubMed, Embase, and CINAHL. Quantitative observational studies investigating the associations between COC and polypharmacy and/or COC and MARO by applying multivariate regression analysis techniques were eligible. Qualitative or experimental studies were not included. Information on the definition and operationalization of COC, polypharmacy, and MARO and reported associations was extracted. COC measures were assigned to the relational, informational, or management dimension of COC and further classified as objective standard, objective non-standard, or subjective. Risk of bias was assessed by using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.
Results
Twenty-seven studies were included. Overall, substantial differences existed in terms of the COC dimensions and related COC measures. Relational COC was investigated in each study, while informational and management COC were only covered among three studies. The most frequent type of COC measure was objective non-standard (n = 16), followed by objective standard (n = 11) and subjective measures (n = 3). The majority of studies indicated that COC is strongly associated with both polypharmacy and MARO, such as potentially inappropriate medication (PIM), potentially inappropriate drug combination (PIDC), drug–drug interaction (DDI), adverse drug events (ADE), unnecessary drug use, duplicated medication, and overdose. More than half of the included studies (n = 15) had a low risk of bias, while five studies had an intermediate and seven studies a high risk of bias.
Conclusions
Differences regarding the methodological quality of included studies as well as the heterogeneity in terms of the operationalization and measurement of COC, polypharmacy, and MARO need to be considered when interpreting the results. Yet, our findings suggest that optimizing COC may be helpful in reducing polypharmacy and MARO. Therefore, COC should be acknowledged as an important risk factor for polypharmacy and MARO, and the importance of COC should be considered when designing future interventions targeting these outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Continuity of care (COC), polypharmacy, and medication appropriateness-related outcomes (MARO) are measured in various ways, without an agreed-upon definition. This complicates study of these concepts and the associations between them. |
Nevertheless, this review finds that COC is strongly associated with both polypharmacy and MARO across a large majority of included studies. |
These results highlight the importance of (a) continuing to study the relationship between COC and polypharmacy/MARO, (b) developing agreed-upon definitions and operationalizations for each concept, and (c) considering COC when designing interventions targeting polypharmacy or MARO. |
1 Background
Due to aging populations and multimorbidity, polypharmacy (taking multiple drugs simultaneously) is an increasing public health problem worldwide [1,2,3,4,5]. Across Europe, approximately one-third of people aged > 65 years are affected by polypharmacy [6]. Because of the heterogeneity of definitions [7] and due to different settings and populations studied, the worldwide prevalence of polypharmacy varies widely between 10 and 90% [8]. Studies have shown that polypharmacy is associated with potentially inappropriate prescribing [9] and several adverse health events [10,11,12]. Accordingly, polypharmacy directly and indirectly affects health care spending and causes avoidable costs [13, 14]. Several interventions have been developed to tackle the growing problem of polypharmacy and associated adverse events; these interventions appear beneficial in terms of improving medication appropriateness-related outcomes (MARO), such as potentially inappropriate prescribing as measured by the Medication Appropriateness Index, Beers’ criteria, and the STOPP/START criteria. Yet, evidence of improvements in clinical outcomes (e.g., reduction of hospital admissions), including patient-reported outcomes, remains inconclusive [15,16,17,18,19].
Suboptimal care transitions and a lack of collaboration between health care providers (e.g., physicians) have been identified as major problems impeding optimal medication management processes and patient safety [20,21,22,23]. In this regard, continuity of care (COC), widely acknowledged as a cornerstone of high-quality care, is highly relevant [24]. According to Haggerty et al. [25], COC comprises three dimensions: relational continuity, representing an ongoing therapeutic relationship between a patient and one or more providers, informational continuity, representing the use of information on past events and personal circumstances to make current care appropriate for each individual, and management continuity, representing a consistent and coherent approach to the management of a health condition that is responsive to a patient’s changing needs. Furthermore, COC can be assessed using three types of measure: ‘objective standard measures’ (e.g., continuity indices), ‘objective non-standard measures’ (e.g., all other quantitative indices of patient–provider contact), and ‘subjective measures’ (patient-reported assessments of continuity) [26].
Evidence suggests that improving COC leads to improved patient-reported outcome measures (e.g., patient satisfaction [26] and quality of life [27]), reduced mortality [28, 29], fewer emergency hospital admissions [30], fewer hospitalizations [31, 32], and decreased healthcare costs [33]. Furthermore, a recent systematic review investigating relational COC in community pharmacies and its effect on patient outcomes found positive effects of higher COC on medication adherence, inappropriate drug use, and the use of other costly services (e.g., visits to the emergency department) [34]. However, there is limited evidence regarding the association of COC with polypharmacy and MARO [24, 26]. Therefore, this study aims (i) to give an overview of how observational studies examining the relationship between COC and polypharmacy on the one hand and COC and MARO on the other operationalize these concepts and (ii) to perform a narrative synthesis of the results of these studies. The former is necessary since COC [25, 35,36,37,38,39], polypharmacy [7], and MARO [40] are defined and measured in various ways, hampering the comparability of results.
2 Methods
This systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) statement [41] (Electronic Supplementary Material [ESM] Tables S4 and S5).
2.1 Search Strategy
We performed a systematic literature search from inception to 06 February 2023 using the databases MEDLINE via PubMed, Embase, and CINAHL via EBSCOhost Web. The search strategy included terms related to COC, polypharmacy, MARO, and relevant MeSH terms. For Embase and CINAHL, the same search terms were used (see supplement 1 in the ESM). Additionally, reference lists of relevant studies were searched manually for further relevant publications. Databases were chosen due to their relevance and the search strategy was developed in accordance with published COC- and MARO-related systematic reviews [16, 19, 27, 34].
2.2 Study Selection
Studies were included if they investigated the relationship between COC and polypharmacy and/or MARO. We included only studies focusing on the continuity of physician care, rather than COC with respect to nurses, pharmacies, or other care providers. Any operationalization of COC, polypharmacy, and MARO was eligible. Only quantitative observational studies (including those using written questionnaires and quantitative interviews) applying multivariate regression analysis techniques were included to ensure that included studies properly controlled for confounding factors. Any experimental and qualitative studies (or reviews of such), editorials, commentaries, conference abstracts, or study protocols were excluded. Experimental studies were excluded as clarifying the operationalization of COC, polypharmacy, and MARO and their relationship in observational studies is a necessary step before interventions targeting COC to improve polypharmacy and MARO can be properly evaluated. The selection was limited to articles published in English and German (see supplement 2 in the ESM). Two investigators (DL and JG) independently screened search results and assessed the eligibility of potentially relevant studies. Discrepancies were resolved by consensus. Another investigator (DG/JW) was involved if consensus could not be reached.
2.3 Data Extraction, Categorization, and Analysis
The following data were extracted from the included studies: information related to study design/analysis, data source (register, claims, administrative and pharmacy data summarized as ‘register/claims data’), country, setting (of exposure), and population. Regarding analyses and outcomes, information on how COC was operationalized was extracted and categorized according to the three dimensions (relational continuity, informational continuity, and management continuity) proposed by Haggerty et al. [25]. Additionally, studies were categorized by their type of COC measure into objective standard measures, objective non-standard measures, and subjective measures according to van Walraven et al. [26]. Key findings of the studies and reported effect sizes, that is, odds ratios (OR), risk ratios (RR), incidence rate ratios (IRR) resulting from regression models, were also extracted (Table 1). Finally, information related to the operationalization of polypharmacy and MARO was extracted (Table 2; Tables S1 and S2 in the ESM). One investigator (DL) performed the data extraction, which was verified by a second investigator (JG). Disagreements were resolved by consensus after discussion.
The results of the included studies were synthesized narratively, since the variety of COC, polypharmacy, and MARO measures as well as differences in reported outcomes and study designs did not allow a quantitative synthesis. For those studies reporting OR, RR, and IRR, we visualized point estimates of the effect sizes as well as reported confidence intervals with forest plots. These plots were grouped by type of COC measure and type of outcome.
2.4 Quality Appraisal
Risk of bias was assessed using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, which comprises 14 criteria and rating guidance [42]. This tool classifies the risk of bias of studies as good (low risk of bias), fair (intermediate risk of bias), or poor (high risk of bias). Two reviewers made independent judgments on each of the items (DL, JG). Disagreements between the two reviewers were resolved by consensus after discussion.
3 Results
3.1 Study Selection
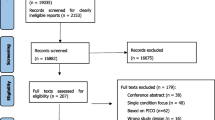
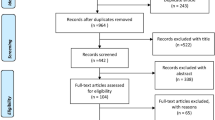
The literature search identified 1984 articles, resulting in 1758 articles after duplicates were removed. After screening titles and abstracts according to the eligibility criteria, we selected 175 articles for full-text review. Full-text articles (n = 160) were excluded with the following reasons: (i) no quantitative association of COC and either polypharmacy or MARO investigated (n = 117), (ii) experimental design or review of interventional studies (n = 39), (iii) conference abstract (no full-text available) (n = 3), (iv) language other than English or German (n = 1). Finally, 27 studies that met the inclusion criteria were included in the narrative synthesis, including 12 studies that were found by searching the reference lists manually (Fig. 1).
PRISMA 2020 flow diagram. Reason 1: No quantitative association of COC and either polypharmacy or MARO investigated, reason 2: experimental design or review of interventional studies, reason 3: conference abstract (no full-text available), reason 4: language other than English or German. COC continuity of care, MARO medication appropriateness-related outcomes
3.2 Study Characteristics and Methodological Findings
Table 1 summarizes the included studies’ main study characteristics and results. The majority of studies (n = 16) investigated the relationship between COC and MARO [43,44,45,46,47, 51, 56,57,58, 60, 61, 63, 64, 66, 68, 69]. Seven studies focused on the relationship between COC and polypharmacy [48, 53, 54, 62, 65, 67], and four studies investigated both the relationship between COC and MARO and between COC and polypharmacy [49, 50, 52, 59].
The included studies were from North America (n = 12), Europe (n = 6), and Asia (n = 9). Most of the studies (n = 9) were from the US [46, 48, 51, 57, 60, 63, 66, 67, 69] and Taiwan (n = 6) [43,44,45, 47, 50, 62]. The population of interest was mostly at least 60 years old. Only five studies included younger patients [45, 48, 51, 57, 58]. Nine studies focused on patients with specific diseases or risks, such as patients with a mental and/or behavioral disorder or dementia [47, 48, 51,52,53,54, 62, 67, 68]. All studies included outpatient data, while only two studies [51, 69] included inpatient data. Sample sizes varied substantially between 384 [60] and 2,318,766 participants [50]. Cross-sectional analyses were performed in 20 [46, 48, 49, 52,53,54, 56,57,58,59,60,61,62,63,64,65,66,67,68,69] and longitudinal analyses in eight studies [43,44,45, 47, 50, 51, 55, 69]. One study performed both cross-sectional and longitudinal analyses [69]. Most studies (n = 19) performed their analyses based on register/claims data [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57, 59, 61, 64, 68]. Five studies used questionnaires/interviews [63, 65,66,67, 69]. One study based its analyses on medical records [60]. A combination of multiple data sources was used by two studies [58, 62]. The main setting (of exposure) was primary care/outpatient. Only two studies included providers from the primary care/outpatient and secondary care/inpatient setting [51, 69]. The following subsections describe methodological characteristics of the included studies, including the measures used to capture COC, polypharmacy, and MARO. Table 2 gives an overview of the frequency of these measures overall and for studies investigating polypharmacy and MARO, respectively.
3.2.1 Operationalization of Continuity of Care (COC)
The most frequent COC dimension investigated was relational continuity, which was considered in every study. Only three studies [48, 57, 66] additionally considered informational continuity and management continuity (Table 1). Regarding the operationalization of COC, substantial differences were observed.
Objective standard COC measures were used by 11 studies. Among those, different COC indices were used to measure relational continuity, such as the Continuity of Care Index (COCI), the Usual Provider of Care (UPC) index, and the Sequential Continuity of Care Index (SECON). The COCI was analyzed in six studies [43,44,45, 47, 50, 52]. The studies differed in terms of their aggregation level. For example, two studies analyzed COCI at the site level in addition to the physician level [43, 45]. Moreover, the variables’ scale of measurement was variously defined as continuous [45, 47], ordinal [44, 45, 50, 52], or binary (e.g., low vs high COCI) [43, 50]. The UPC index was also calculated in six studies [43, 44, 52,53,54, 64]. Two of these studies used the UPC index to conduct supplementary sensitivity analyses beyond their primary COCI-based analyses [43, 44]. Differences in the aggregational level (physician level vs site level) and the variables’ scale of measurement also existed among those studies. Two studies [48, 57] operationalized COC via care density, a proxy measure that may reflect how frequently a patient’s doctors collaborate/share patients. Thus, care density corresponds to better communication and information sharing between the patient’s care team, forming a social network of providers [70]. This was the only COC measure identified that represents informational and management COC. The SECON was only used by one study that also calculated the COCI and the UPC index [52]. Multiple objective standard measures of COC were used by three studies [43, 44, 52] (Table 2; Tables S1 and S2 in the ESM).
Among studies using objective non-standard measures of COC (n = 16), the majority (n = 11) used the number of prescribers [46, 48, 51, 55, 56, 59,60,61, 63, 68, 69] to measure COC, with a high number of prescribers indicating low COC. Further measures were the number of treating physicians [49, 61], the number of providers [57, 67], the number of specialties [58], the tendency to visit multiple providers [62], and having a single primary care physician [61]. Exposure variables were treated as binary, ordinal, or continuous (Table 2; Tables S1 and S2 in the ESM).
Subjective measures of COC were used by three studies [65, 66, 69] (Table 2). In particular, patients were asked if they have a regular physician [65], whether they usually see the same physician [69], or whether they experienced a gap in care coordination [66]. These COC measures were treated as binary variables (yes vs no) (Tables S1 and S2, see ESM). Overall, a combination of the different types of COC measures was used by three studies [48, 57, 69].
3.2.2 Operationalization of Polypharmacy
Polypharmacy was mostly defined as having five or more medications prescribed (binary variable) [49, 50, 52, 55, 59, 62, 65, 67]. Some studies (additionally) included extreme/excessive polypharmacy (≥10 medications prescribed) [50, 52,53,54,55, 62]. One study operationalized multiclass psychotropic polypharmacy as taking two or more psychotropic medications from different drug classes for 60 days or more [48]. Observational periods varied from 2 weeks to 1 year; two studies also considered persistent (>181 days) polypharmacy [50, 62] (Table 2; Table S1, see ESM).
3.2.3 Operationalization of Medication Appropriateness-Related Outcomes (MARO)
Overall, seven categories of MARO were investigated: Potentially inappropriate medication (PIM) [44, 46, 47, 49, 52, 56, 59, 64, 69], drug–drug interaction (DDI) [45, 50, 57, 64, 66, 68], adverse drug events (ADE) [58, 63], duplicated medication [43, 44], unnecessary drug use [60], overdose [51], and potential inappropriate drug combination (PIDC) [61] (Table 2).
Regarding the operationalization of PIM, different versions of the Beers criteria [71] were applied [46, 47, 64]. Other instruments were used, such as the Japanese STOPP-J list [59], the Norwegian General Practice (NORGEP) criteria, which are based on the Beers criteria [56], the German PRISCUS list [49], and the STOPP/START criteria [52]. PIM was always analyzed by using a binary (yes vs no) variable. Concerning DDI, the outcome variable was dichotomized (yes vs no) in all but one included study, which treated DDI as a continuous variable [45]. PIDC, as used by Tamblyn et al. [61], is a combination of PIM and DDI, identified by an expert review. Duplicated medications were used as outcomes by Cheng and Chen [43] and Chu et al. [44]. ADE were defined as either the presence of an ADE-specific code [58] or as a binary (yes vs no) outcome self-reported by the study participants [63]. One study [60] measured unnecessary drug use based on the Medication Appropriateness Index [72]. Finally, overdose as an outcome was defined as the occurrence of one or more medical claims containing a diagnosis code for opioid or benzodiazepine poisoning on a person-day of opioid-benzodiazepine overlap [51] (Table S2, see ESM).
3.3 Association Between COC and Polypharmacy
Studies using objective standard measures of COC [48, 50, 52,53,54] found mixed effects concerning the association between COC and polypharmacy (Table 1). For example, higher COC (highest quartile, ref.: lowest quartile) was not associated with polypharmacy but with a reduced risk of extreme polypharmacy [52]. Two studies by Guilcher et al. [53, 54] also showed a significant negative association between COC and polypharmacy. Furthermore, COC (care density) was associated with the likelihood of receiving psychotropic polypharmacy. However, this relationship between COC (care density) and psychotropic polypharmacy varied depending on the type of physicians involved in the care team, and a significant negative relationship between COC (care density) and psychotropic polypharmacy was only observed among patients with only PCPs involved in their care teams, while a significant positive relationship was observed among patients who had both PCPs and specialists involved in their care team [48]. Weng et al. [50] showed that the proportion of patients with polypharmacy was significantly lower in a high COC group (87.80%) compared with a low COC group (94.29%) and that higher COC was related to fewer DDI events. This latter effect was partially mediated by polypharmacy. Fig. 2 shows the associations between COC and polypharmacy in studies using objective standard COC measures.
Association between COC and polypharmacy for objective standard COC measures. Solid green line indicates significant negative association between COC and polypharmacy; dashed red line indicates non-significant negative association; blue dotted line indicates significant positive association; [50] was not visualized, as results were not reported as OR, RR, or IRR; [54] uses low COC as the reference category. Therefore, an RR of 1.07 indicates a negative relationship between high COC and polypharmacy. *OR for care teams of PCPs only; **OR for care teams of specialists only; ***OR for care teams with both PCPs and specialists. COC continuity of care, COCI Continuity of Care Index (physician level), IRR incidence rate ratio, MARO medication appropriateness-related outcomes, OR odds ratio, PCP primary care physician/practitioner, RR risk ratio, SECON sequential continuity of care, UPC usual provider of care
All studies using objective non-standard COC measures (e.g., number of prescribers/providers/treating physicians) [48, 49, 55, 59, 62, 67] demonstrated associations between COC and (different levels of) polypharmacy. Regarding polypharmacy (≥ 5 medications) [49, 55, 59, 62, 67], studies consistently showed a significant association with COC. For instance, one study demonstrated that higher COC (lower number of treating physicians) is a predictor of polypharmacy (independent of multimorbidity) in men and women above the age of 60 years [49]. Regarding subjective COC measures, one study showed that patients reporting low COC (not having a regular physician) are more than twice as likely to be taking five or more prescribed drugs than those patients with high COC (having a regular physician) [65]. Figure 3 shows the associations between COC and polypharmacy in studies using objective non-standard or subjective COC measures.
Association between COC and polypharmacy for objective non-standard and subjective COC measures. For [49], only the OR for 2 vs 1 physician among women was visualized. The association between the number of treating physicians and polypharmacy was significantly positive in all other subgroups. Solid green line indicates significant negative association between COC and polypharmacy. COC continuity of care, OR odds ratio
3.4 Association Between COC and MARO
Studies using objective standard measures of COC [43,44,45, 47, 50, 57, 64] to investigate the association of COC with PIM [44, 47, 52, 64], DDI [45, 50, 57, 64], and medication duplication [43, 44] demonstrated negative relationships (Table 1). In terms of PIM, however, one study [52] showed mixed results (significant or non-significant negative associations) depending on the type of analysis. Figure 4 shows the associations between COC and MARO in studies using objective standard COC measures.
Association between COC and MARO for objective standard COC measures. Solid green line indicates significant negative association between COC and MARO; dashed red line indicates non-significant negative association. [43] was not visualized, as results were not reported as OR, RR, or IRR. COC continuity of care, COCI Continuity of Care Index (physician level), DDI drug–drug interaction, HF heart failure, IRR incidence rate ratio, MARO medication appropriateness-related outcomes, OR odds ratio, PIM potentially inappropriate medication, RR risk ratio, SECON sequential continuity of care, UPC usual provider of care
Objective non-standard COC measures were used by 12 studies [46, 49, 51, 56,57,58,59,60,61, 63, 68, 69]. Regarding PIM, most studies revealed negative associations with COC [46, 49, 56, 59]. However, one study showed that having low COC (high number of prescribers) was not significantly associated with PIM [69]. DDI [57, 68], ADE [58, 63], unnecessary drug use [60], overdose [51], and PIDC [61] were found to be negatively associated with COC. Thus, the more prescribers/providers involved in the care process (representing lower COC), the higher the likelihood of inappropriate prescribing. Subjective COC measures were used by two studies [66, 69]. These studies also identified negative associations of COC (gap in care coordination) and DDI [66] and COC (usually seeing the same physician) and PIM [69]. Figure 5 shows the associations between COC and MARO in studies using objective non-standard or subjective COC measures.
Association between COC and MARO for objective non-standard and subjective COC measures. Solid green line significant negative association between COC and MARO; red dashed line non-significant negative association. For [46], the OR for having 2 vs 1 prescriber was visualized (a significant negative association between COC and PIM was also found for 3 and 4+ vs 1 prescriber). For [49], only the OR for 2 vs 1 physician among women was visualized. The association between the number of treating physicians and polypharmacy was significantly positive in all other subgroups. For [56], the OR for 3–4 vs 1–2 prescribers was visualized (a significant negative association between COC and PIM was also found for 5 + vs 1–2 prescribers). For [69], only the longitudinal model was visualized. ADE adverse drug event, COC continuity of care, COCI Continuity of Care Index, DDI drug–drug interaction, MARO medication appropriateness-related outcomes, OR odds ratio, PIDC potential inappropriate drug combination, PIM potentially inappropriate medication
3.5 Risk of Bias Assessment
Overall, 15 studies [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] had a low risk of bias, five studies [58,59,60,61,62] had an intermediate risk of bias, and seven studies [63,64,65,66,67,68,69] were deemed to have a high risk of bias (Table S3, see ESM). Among studies at high risk of bias, common problems were that the exposure measures (criteria 9) and outcome measures (criteria 11) were not clearly defined, valid, reliable, and implemented consistently across all study participants, that the exposure was not measured before the outcome(s) (criteria 6), or that the exposure was not assessed more than once over time (criteria 10). Loss to follow-up (criteria 13) was deemed not applicable (N/A) to the included studies, as all studies were retrospective.
4 Discussion
This systematic review had two aims. First, we aimed to explore how COC, polypharmacy and MARO are defined, operationalized, and measured in the included studies. Second, we aimed to investigate the relationship between COC and polypharmacy and MARO.
4.1 Methodological Findings: Measuring COC
COC is a multi-faceted concept with various measures available to researchers [38, 73]. However, different COC measures reflect the dimensions of relational, informational, and management continuity to various degrees. We found that the majority of included studies used objective non-standard measures, mainly the number of prescribers, while a significant minority used objective standard measures, with only three studies using subjective measures.
Objective non-standard measures are typically simple to compute but are only partially adequate for measuring COC. First, these measures quantify the amount of patient-provider interaction (e.g., the number of particular patient-doctor encounters [26]) without considering the distribution of these interactions. However, this distribution is important to relational continuity. Second, objective non-standard measures do not adequately capture informational or management continuity. For instance, it is not plausible to consider a patient with a moderate number of prescribers who do not effectively communicate and share information about the patient to have a higher COC than a patient with a somewhat larger but well-connected group of prescribers who adhere to joint treatment plans.
Similarly, objective standard measures were also mainly used to measure relational continuity. However, these measures may be better suited to measuring all COC dimensions than objective non-standard measures. First, they can capture aspects of relational continuity beyond the mere number of providers or prescribers, such as the distribution of visits to different providers. Second, while they were not commonly used, objective standard measures that are capable of measuring informational and management COC do exist (e.g., care density). When measuring relational continuity, included studies used a variety of COC indices and different cut-off values for high and low COC even when the same index was used. In fact, for the COCI, there is no agreed-upon cut-off value for high and low continuity [74]. This makes it more difficult to compare results between studies.
Few studies used subjective COC measures [53, 55, 68]. While these patient-reported measures are more susceptible to bias than objective COC measures, subjective measures are a valuable supplement to objective measures relying on claims data. Overall, our findings on utilized measures of COC are consistent with other studies showing that objective COC measures referring to relational continuity are most commonly used [24, 26, 33].
The results indicate that there is no agreed-upon approach for measuring COC as a multi-faceted concept in the context of polypharmacy and medication appropriateness. Future research in this area should aim to measure all three dimensions of COC and use multiple COC measures to make comparing measures and their results easier. This means researchers should ensure that the measures used cover all COC dimensions. For example, studies may use a relational COC measure in concert with an informational and management COC measure such as care density or an appropriate subjective measure. However, it remains unclear to what extent these measures appropriately capture informational and management continuity. For instance, care density is only a surrogate measure for care communication and collaboration, based on the premise that certain aspects of coordination may be reflected and/or facilitated by patients seeing physicians whose patient panels significantly overlap [75]. It should also be taken into account that a high number of patients shared between physicians does not indicate that these physicians necessarily exchange (sufficient) information about their patients [75]. Therefore, care density is only able to examine conditions that are more or less favorable toward management and informational continuity (care coordination) [75]. This highlights the need for developing, validating, and using new COC measures referring to the management and information dimension of COC. In addition, researchers should use a combination of different types of COC measures, even within COC dimensions. This means using and comparing multiple COC indices, including those with different methodological approaches (e.g., dispersion, density, or sequence of doctor visits [33, 38]) when utilizing objective standard measures. This was only done in three studies [43, 44, 52]. Furthermore, many objective standard measures of relational continuity are calculated from values commonly used as objective non-standard measures. In such cases, researchers should report and compare the values and associations with polypharmacy/MARO for both the objective standard and objective non-standard measures. This was only done in two studies [48, 57]. Finally, sensitivity analyses may be appropriate when using COC indices to acknowledge methodological differences in operationalization.
Similar conclusions can be drawn from an analysis of the data types used by the included studies. A large majority of studies used claims data or similar data types to measure continuity, allowing researchers to reach very large sample sizes and compute objective standard and objective non-standard measures. However, COC indices based on claims data cannot fully capture the multiple dimensions of COC [33, 76]. A small number of studies used survey data to measure continuity. While survey data alone is also inadequate to capture all three dimensions of continuity [77], future studies should use appropriate survey-based measures to complement claims-based measures to capture COC in all its facets [76]. This is particularly important when investigating the association between COC and polypharmacy or MARO, as research has shown discrepancies between COC measured through survey data and claims data [78].
4.2 Methodological Findings: Measuring Polypharmacy and MARO
Substantial differences existed concerning how polypharmacy and MARO were operationalized and analyzed. While most of the studies defined polypharmacy using a numerical threshold of more than five drugs, which is commonly used in the literature [7], studies differed concerning the timeframe in which the numerical threshold could be reached. For instance, some studies analyzed the number of drugs within a 1-year period, while others focused on the day of maximum prescriptions. This finding aligns with current research showing that polypharmacy continues to lack a universally accepted definition [7, 79]. However, operationalizations based solely on numerical data do not adequately capture the complexity of the problem and make it difficult to assess the safety and appropriateness of drug therapy in clinical practice. For instance, using multiple medications is not necessarily harmful and associated with adverse health effects but may even be entirely reasonable and appropriate for some (multimorbid) patients. Thus, the use of strict numerical cut-offs to measure and operationalize polypharmacy has been criticized. Accordingly, some authors propose distinguishing between appropriate and inappropriate polypharmacy and placing more emphasis on qualifying the term polypharmacy rather than quantifying it [80, 81]. However, there is little evidence on how to distinguish between appropriate and inappropriate polypharmacy [82]. In the absence of a uniform definition, studies should continue to use the five-drug threshold to ensure comparability across studies. However, researchers should perform sensitivity analyses with higher or lower thresholds to test the robustness of their results. Finally, future research should work toward developing a useable definition of ‘inappropriate polypharmacy’, moving away from strictly quantitative definitions. Regarding the operationalization of MARO, future research should aim to use agreed-upon definitions and operationalizations (particularly concerning DDI) to ensure the comparability of results.
4.3 Methodological Findings: Risk of Bias
Most studies had a low risk of bias (n = 15/24). These studies examined exposure and outcome based on register/claims data. Studies using questionnaire/interview-based data had a higher risk of bias, indicating that large claims databases can be useful for analyzing COC. However, this is due to the subjective measures used in the included questionnaire/interview-based studies and does not show that subjective measures are generally inappropriate for measuring COC. Instead, these results again highlight the importance of developing suitable and agreed-upon subjective measures for COC, especially the informational and management dimensions.
Additionally, several studies had a higher risk of bias because they failed to address time-dependent bias, a common methodological flaw in COC research [26, 83]. Appropriate accounting for the relative timing of COC and outcomes was ensured by only 11 studies [43,44,45, 47, 50,51,52, 55, 60, 65, 69]. In terms of the study design, longitudinal studies had rather good quality compared with cross-sectional studies. However, well conducted, cross-sectional studies did exist [46, 48, 49, 52,53,54, 56, 57]. Overall, differences regarding the methodological quality of included studies need to be considered when interpreting the results. Future studies should ensure that COC is measured before outcomes, or at least address the issue of relative timing with appropriate methods; aim to have a longitudinal design to investigate the long-term effects of COC on outcomes; and use register/claims data to reduce potential recall bias and to expand the study period at comparatively low cost.
4.4 Empirical Findings
Yet, despite the conceptual variety and differences in quality between studies, our findings suggest a strong association between COC and polypharmacy and between COC and MARO. These results yield that (i) lower COC increases the chance of polypharmacy and (ii) lower COC increases the chance of MARO such as PIM, PIDC, DDI, ADE, unnecessary drug use, medication duplication, and overdose. As shown by Weng et al. [50], the relationship between COC and inappropriate prescribing (DDI) is mediated by polypharmacy, indicating that polypharmacy itself is an important risk factor for several drug-related adverse events [84].
Our results contribute to the findings of Choi and Lee [34], who investigated the relationship of relational COC between patients and community pharmacy (CP) pharmacists. They showed that a high degree of relational COC between patients and CP pharmacists was associated with improved medication adherence. Patients who had visited a single pharmacy were more adherent to their medication regimen compared with those visiting multiple pharmacies. Moreover, a high level of relational continuity could lower inappropriate drug use and emergency department visits caused by adverse drug reactions [34]. Other studies also showed the importance of doctor–patient COC for safer medication management, demonstrating that higher COC was associated with higher medication adherence and compliance [85,86,87].
4.5 Implications for Research and Practice
Our findings have significant implications for health care research and practice. Concerning the operationalization and measurement of COC, our methodological findings highlight that researchers should (i) ensure that all three dimensions of COC (relational, informational, and management continuity) are covered by the COC measures used, (ii) use and compare different COC measures of the same type, (iii) use a combination of subjective and objective COC measures, and (iv) draw from a combination of claims data and patient-reported survey data when doing so. These steps will help researchers better understand and use the various tools available for measuring COC. In particular, future research should aim to identify or develop an appropriate and agreed-upon operationalization of COC, polypharmacy, and MARO to ensure the comparability of results. Researchers investigating the link between COC and outcomes such as polypharmacy or MARO should use longitudinal study designs where possible and give particular regard to the relative timing of exposures and outcomes.
Following these recommendations may also allow future research to improve health care practice regarding COC. Our findings indicate that low COC is a significant risk factor for polypharmacy and MARO, highlighting the need for appropriate interventions to improve COC. However, designing and targeting these interventions will require a more detailed understanding of the underlying causal links between the three dimensions of continuity and outcomes, such as polypharmacy or MARO. Overall, health care providers and researchers involved in intervention planning should acknowledge low COC as an important risk factor for polypharmacy/MARO and consider all three dimensions of COC when designing interventions. This contributes to the findings of Facchinetti et al. on the importance of developing interventions that address all continuity dimensions simultaneously [88].
4.6 Limitations
While several COC-related systematic reviews have been published, including various health-related outcomes [26, 28, 29, 32, 89, 90], this review is the first to explore doctor–patient COC in polypharmacy and medication management. Nevertheless, some limitations of this review need to be considered. First, there was substantial heterogeneity between studies regarding the measurement and operationalization of exposure and outcomes variables. This allowed us to analyze the methodological approaches to measuring COC, polypharmacy, and MARO used by included studies, but complicated the comparison of empirical findings between different studies. Second, some studies had strong methodological flaws, such as the relative timing of the measurement of exposure and outcomes. Third, in terms of the generalizability of the results, population and health system-related differences need to be considered. However, despite different populations and health care systems studied, the empirical findings of the included studies were quite consistent. Fourth, the literature search was restricted to articles published in English and German. As a significant minority of included studies were from non-English and non-German speaking countries, it is likely that there are further relevant studies that we did not include. Fifth, we included only quantitative studies. Therefore, qualitative approaches to exploring the relationship between COC and polypharmacy/MARO could not be considered. Sixth, due to different operationalizations of MARO, the search strategy may not have been sufficient to identify all relevant studies on this topic. Seventh, another limitation is that this review and its methods were not registered in a review study registry (e.g., PROSPERO) before it was conducted. However, the methodological aspects were pre-specified in the work process and described transparently in this article. Finally, a meta-analysis of effect sizes across studies could not be conducted, given the heterogeneity of study characteristics.
5 Conclusion
This systematic review summarized evidence supporting the negative associations between COC and polypharmacy and between COC and MARO. Despite differences in the operationalization of COC, polypharmacy, and MARO, our findings suggest that improving COC is a promising approach to managing polypharmacy and preventing inappropriate prescribing. However, further research is necessary to develop agreed-upon definitions and operationalizations of the concepts involved, including operationalization of COC that covers all continuity dimensions and an appropriate definition of inappropriate polypharmacy. This will allow researchers and practitioners to design interventions targeting the specific causal links between different continuity dimensions and outcomes, such as inappropriate polypharmacy or MARO.
References
Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug–drug interactions: population database analysis 1995–2010. BMC Med. 2015;13:74. https://doi.org/10.1186/s12916-015-0322-7.
Hovstadius B, Hovstadius K, Astrand B, Petersson G. Increasing polypharmacy—an individual-based study of the Swedish population 2005–2008. BMC Clin Pharmacol. 2010;10:16. https://doi.org/10.1186/1472-6904-10-16.
Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–31. https://doi.org/10.1001/jama.2015.13766.
Moßhammer D, Haumann H, Mörike K, Joos S. Polypharmacy—an upward trend with unpredictable effects. Dtsch Arztebl Int. 2016;113:627–33. https://doi.org/10.3238/arztebl.2016.0627.
van den Akker M, Vaes B, Goderis G, van Pottelbergh G, de Burghgraeve T, Henrard S. Trends in multimorbidity and polypharmacy in the Flemish-Belgian population between 2000 and 2015. PLoS ONE. 2019;14:e0212046. https://doi.org/10.1371/journal.pone.0212046.
Midão L, Giardini A, Menditto E, Kardas P, Costa E. Polypharmacy prevalence among older adults based on the survey of health, ageing and retirement in Europe. Arch Gerontol Geriatr. 2018;78:213–20. https://doi.org/10.1016/j.archger.2018.06.018.
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. https://doi.org/10.1186/s12877-017-0621-2.
Khezrian M, McNeil CJ, Murray AD, Myint PK. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther Adv Drug Saf. 2020;11:1–10. https://doi.org/10.1177/2042098620933741.
Tommelein E, Mehuys E, Petrovic M, Somers A, Colin P, Boussery K. Potentially inappropriate prescribing in community-dwelling older people across Europe: a systematic literature review. Eur J Clin Pharmacol. 2015;71:1415–27. https://doi.org/10.1007/s00228-015-1954-4.
Davies LE, Spiers G, Kingston A, Todd A, Adamson J, Hanratty B. Adverse outcomes of polypharmacy in older people: systematic review of reviews. J Am Med Dir Assoc. 2020;21:181–7. https://doi.org/10.1016/j.jamda.2019.10.022.
Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62:2261–72. https://doi.org/10.1111/jgs.13153.
Gnjidic D, Tinetti M, Allore HG. Assessing medication burden and polypharmacy: finding the perfect measure. Expert Rev Clin Pharmacol. 2017;10:345–7. https://doi.org/10.1080/17512433.2017.1301206.
Chiatti C, Bustacchini S, Furneri G, Mantovani L, Cristiani M, Misuraca C, Lattanzio F. The economic burden of inappropriate drug prescribing, lack of adherence and compliance, adverse drug events in older people: a systematic review. Drug Saf. 2012;35(Suppl 1):73–87. https://doi.org/10.1007/BF03319105.
Heider D, Matschinger H, Meid AD, Quinzler R, Adler J-B, Günster C, et al. The impact of potentially inappropriate medication on the development of health care costs and its moderation by the number of prescribed substances. Results of a retrospective matched cohort study. PLoS ONE. 2018;13:e0198004. https://doi.org/10.1371/journal.pone.0198004.
Anderson LJ, Schnipper JL, Nuckols TK, Shane R, Sarkisian C, Le MM, Pevnick JM. A systematic overview of systematic reviews evaluating interventions addressing polypharmacy. Am J Health Syst Pharm. 2019;76:1777–87. https://doi.org/10.1093/ajhp/zxz196.
Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2018;9:CD008165. https://doi.org/10.1002/14651858.CD008165.pub4.
Johansson T, Abuzahra ME, Keller S, Mann E, Faller B, Sommerauer C, et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82:532–48. https://doi.org/10.1111/bcp.12959.
Cooper JA, Cadogan CA, Patterson SM, Kerse N, Bradley MC, Ryan C, Hughes CM. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015;5:e009235. https://doi.org/10.1136/bmjopen-2015-009235.
Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, Hughes C. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2014. https://doi.org/10.1002/14651858.CD008165.pub3.
Kim J, Parish AL. Polypharmacy and medication management in older adults. Nurs Clin North Am. 2017;52:457–68. https://doi.org/10.1016/j.cnur.2017.04.007.
Sponsler KC, Neal EB, Kripalani S. Improving medication safety during hospital-based transitions of care. CCJM. 2015;82:351–60. https://doi.org/10.3949/ccjm.82a.14025.
Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297:831–41. https://doi.org/10.1001/jama.297.8.831.
Lu CY, Roughead E. Determinants of patient-reported medication errors: a comparison among seven countries. Int J Clin Pract. 2011;65:733–40. https://doi.org/10.1111/j.1742-1241.2011.02671.x.
Beadles CA, Voils CI, Crowley MJ, Farley JF, Maciejewski ML. Continuity of medication management and continuity of care: conceptual and operational considerations. SAGE Open Med. 2014;2:2050312114559261. https://doi.org/10.1177/2050312114559261.
Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ. 2003;327:1219–21. https://doi.org/10.1136/bmj.327.7425.1219.
van Walraven C, Oake N, Jennings A, Forster AJ. The association between continuity of care and outcomes: a systematic and critical review. J Eval Clin Pract. 2010;16:947–56. https://doi.org/10.1111/j.1365-2753.2009.01235.x.
Chen H-M, Tu Y-H, Chen C-M. Effect of continuity of care on quality of life in older adults with chronic diseases: a meta-analysis. Clin Nurs Res. 2017;26:266–84. https://doi.org/10.1177/1054773815625467.
Baker R, Freeman GK, Haggerty JL, Bankart MJ, Nockels KH. Primary medical care continuity and patient mortality: a systematic review. Br J Gen Pract. 2020;70:e600–11. https://doi.org/10.3399/bjgp20X712289.
Pereira Gray DJ, Sidaway-Lee K, White E, Thorne A, Evans PH. Continuity of care with doctors-a matter of life and death? A systematic review of continuity of care and mortality. BMJ Open. 2018;8:e021161. https://doi.org/10.1136/bmjopen-2017-021161.
Ionescu-Ittu R, McCusker J, Ciampi A, Vadeboncoeur A-M, Roberge D, Larouche D, et al. Continuity of primary care and emergency department utilization among elderly people. CMAJ. 2007;177:1362–8. https://doi.org/10.1503/cmaj.061615.
Menec VH, Sirski M, Attawar D, Katz A. Does continuity of care with a family physician reduce hospitalizations among older adults? J Health Serv Res Policy. 2006;11:196–201. https://doi.org/10.1258/135581906778476562.
Kao Y-H, Lin W-T, Chen W-H, Wu S-C, Tseng T-S. Continuity of outpatient care and avoidable hospitalization: a systematic review. Am J Manag Care. 2019;25:e126–34.
Nicolet A, Al-Gobari M, Perraudin C, Wagner J, Peytremann-Bridevaux I, Marti J. Association between continuity of care (COC), healthcare use and costs: what can we learn from claims data? A rapid review. BMC Health Serv Res. 2022;22:658. https://doi.org/10.1186/s12913-022-07953-z.
Choi E, Lee I-H. Relational continuity of care in community pharmacy: a systematic review. Health Soc Care Community. 2021. https://doi.org/10.1111/hsc.13428.
Bachrach LL. Continuity of care for chronic mental patients: a conceptual analysis. Am J Psychiatry. 1981;138:1449–56. https://doi.org/10.1176/ajp.138.11.1449.
Freeman GK, Olesen F, Hjortdahl P. Continuity of care: an essential element of modern general practice? Fam Pract. 2003;20:623–7. https://doi.org/10.1093/fampra/cmg601.
Gulliford M, Naithani S, Morgan M. What is ‘continuity of care’? J Health Serv Res Policy. 2006;11:248–50. https://doi.org/10.1258/135581906778476490.
Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev. 2006;63:158–88. https://doi.org/10.1177/1077558705285294.
Saultz JW. Defining and measuring interpersonal continuity of care. Ann Fam Med. 2003;1:134–43. https://doi.org/10.1370/afm.23.
Kaufmann CP, Tremp R, Hersberger KE, Lampert ML. Inappropriate prescribing: a systematic overview of published assessment tools. Eur J Clin Pharmacol. 2014;70:1–11. https://doi.org/10.1007/s00228-013-1575-8.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160.
National Heart Lung and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 5 Jan 2022.
Cheng S-H, Chen C-C. Effects of continuity of care on medication duplication among the elderly. Med Care. 2014;52:149–56. https://doi.org/10.1097/MLR.0000000000000042.
Chu H-Y, Chen C-C, Cheng S-H. Continuity of care, potentially inappropriate medication, and health care outcomes among the elderly: evidence from a longitudinal analysis in Taiwan. Med Care. 2012;50:1002–9. https://doi.org/10.1097/MLR.0b013e31826c870f.
Guo J-Y, Chou Y-J, Pu C. Effect of continuity of care on drug–drug interactions. Med Care. 2017;55:744–51. https://doi.org/10.1097/MLR.0000000000000758.
Holmes HM, Luo R, Kuo Y-F, Baillargeon J, Goodwin JS. Association of potentially inappropriate medication use with patient and prescriber characteristics in Medicare Part D. Pharmacoepidemiol Drug Saf. 2013;22:728–34. https://doi.org/10.1002/pds.3431.
Lai Y-R, Yang Y-S, Tsai M-L, Lu Y-L, Kornelius E, Huang C-N, Chiou J-Y. Impact of potentially inappropriate medication and continuity of care in a sample of Taiwan elderly patients with diabetes mellitus who have also experienced heart failure. Geriatr Gerontol Int. 2016;16:1117–26. https://doi.org/10.1111/ggi.12606.
Medhekar R, Fujimoto K, Aparasu RR, Bhatara VS, Johnson ML, Alonzo JP, et al. Physician care coordination and the use of psychotropic polypharmacy in the management of pediatric mental disorders. J Manag Care Spec Pharm. 2019;25:29–38. https://doi.org/10.18553/jmcp.2019.25.1.029.
Tetzlaff F, Singer A, Swart E, Robra B-P, Herrmann MLH. Polypharmazie in der nachstationären Versorgung: Eine Analyse mit Daten der AOK Sachsen-Anhalt. [Polypharmacy after Discharge from Hospital: An Analysis Using Data of the Statutory Health Insurance (AOK) of Saxony-Anhalt]. Gesundheitswesen. 2018;80:557–63. https://doi.org/10.1055/s-0042-113599.
Weng Y-A, Deng C-Y, Pu C. Targeting continuity of care and polypharmacy to reduce drug–drug interaction. Sci Rep. 2020;10:21279. https://doi.org/10.1038/s41598-020-78236-y.
Chua K-P, Brummett CM, Ng S, Bohnert ASB. Association between receipt of overlapping opioid and benzodiazepine prescriptions from multiple prescribers and overdose risk. JAMA Netw Open. 2021;4:e2120353. https://doi.org/10.1001/jamanetworkopen.2021.20353.
Delgado J, Evans PH, Gray DP, Sidaway-Lee K, Allan L, Clare L, et al. Continuity of GP care for patients with dementia: impact on prescribing and the health of patients. Br J Gen Pract. 2022;72:e91–8. https://doi.org/10.3399/BJGP.2021.0413.
Guilcher SJT, Hogan M-E, Calzavara A, Hitzig SL, Patel T, Packer T, Lofters AK. Prescription drug claims following a traumatic spinal cord injury for older adults: a retrospective population-based study in Ontario, Canada. Spinal Cord. 2018;56:1059–68. https://doi.org/10.1038/s41393-018-0174-z.
Guilcher SJT, Hogan M-E, McCormack D, Calzavara AJ, Hitzig SL, Patel T, et al. Prescription medications dispensed following a nontraumatic spinal cord dysfunction: a retrospective population-based study in Ontario, Canada. Spinal Cord. 2020;59:132–40. https://doi.org/10.1038/s41393-020-0511-x.
Kann IC, Lundqvist C, Lurås H. Polypharmacy among the elderly in a list-patient system. Drugs Real World Outcomes. 2015;2:193–8. https://doi.org/10.1007/s40801-015-0036-3.
Nyborg G, Straand J, Brekke M. Inappropriate prescribing for the elderly—a modern epidemic? Eur J Clin Pharmacol. 2012;68:1085–94. https://doi.org/10.1007/s00228-012-1223-8.
Ong M-S, Olson KL, Chadwick L, Liu C, Mandl KD. The impact of provider networks on the co-prescriptions of interacting drugs: a claims-based analysis. Drug Saf. 2017;40:263–72. https://doi.org/10.1007/s40264-016-0490-1.
Calderón-Larrañaga A, Poblador-Plou B, González-Rubio F, Gimeno-Feliu LA, Abad-Díez JM, Prados-Torres A. Multimorbidity, polypharmacy, referrals, and adverse drug events: are we doing things well? Br J Gen Pract. 2012;62:e821–6. https://doi.org/10.3399/bjgp12X659295.
Fujie K, Kamei R, Araki R, Hashimoto K. Prescription of potentially inappropriate medications in elderly outpatients: a survey using 2015 Japanese guidelines. Int J Clin Pharm. 2020;42:579–87. https://doi.org/10.1007/s11096-020-00967-9.
Hajjar ER, Hanlon JT, Sloane RJ, Lindblad CI, Pieper CF, Ruby CM, et al. Unnecessary drug use in frail older people at hospital discharge. J Am Geriatr Soc. 2005;53:1518–23. https://doi.org/10.1111/j.1532-5415.2005.53523.x.
Tamblyn RM, McLeod PJ, Abrahamowicz M, Laprise R. Do too many cooks spoil the broth? Multiple physician involvement in medical management of elderly patients and potentially inappropriate drug combinations. CMAJ. 1996;154:1177–84.
Chan D-CD, Hao Y-T, Wu S-C. Polypharmacy among disabled Taiwanese elderly: a longitudinal observational study. Drugs Aging. 2009;26:345–54. https://doi.org/10.2165/00002512-200926040-00005.
Green JL, Hawley JN, Rask KJ. Is the number of prescribing physicians an independent risk factor for adverse drug events in an elderly outpatient population? Am J Geriatr Pharmacother. 2007;5:31–9. https://doi.org/10.1016/j.amjopharm.2007.03.004.
Jang S, Jeong S, Jang S. Patient- and prescriber-related factors associated with potentially inappropriate medications and drug–drug interactions in older adults. J Clin Med. 2021. https://doi.org/10.3390/jcm10112305.
Tsuji-Hayashi Y, Fukuhara S, Green J, Kurokawa K. Use of prescribed drugs among older people in Japan: association with not having a regular physician. J Am Geriatr Soc. 1999;47:1425–9. https://doi.org/10.1111/j.1532-5415.1999.tb01561.x.
Kern LM, Reshetnyak E, Colantonio LD, Muntner PM, Rhodes JD, Casalino LP, et al. Association between patients’ self-reported gaps in care coordination and preventable adverse outcomes: a cross-sectional survey. J Gen Intern Med. 2020;35:3517–24. https://doi.org/10.1007/s11606-020-06047-y.
Bazargan M, Smith J, Movassaghi M, Martins D, Yazdanshenas H, Salehe Mortazavi S, Orum G. Polypharmacy among underserved older African American adults. J Aging Res. 2017;2017:6026358. https://doi.org/10.1155/2017/6026358.
Bjerrum L, Gonzalez Lopez-Valcarcel B, Petersen G. Risk factors for potential drug interactions in general practice. Eur J Gen Pract. 2008;14:23–9. https://doi.org/10.1080/13814780701815116.
Hanlon JT, Fillenbaum GG, Schmader KE, Kuchibhatla M, Horner RD. Inappropriate drug use among community-dwelling elderly. Pharmacotherapy. 2000;20:575–82. https://doi.org/10.1592/phco.20.6.575.35163.
Pollack CE, Lemke KW, Roberts E, Weiner JP. Patient sharing and quality of care: measuring outcomes of care coordination using claims data. Med Care. 2015;53:317–23. https://doi.org/10.1097/MLR.0000000000000319.
American Geriatrics Society. Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–94. https://doi.org/10.1111/jgs.15767.
Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045–51. https://doi.org/10.1016/0895-4356(92)90144-C.
Reid R, Haggerty J, McKendry R. Defusing the confusion: concepts and measures of continuity of health care. Final Report. 2002. Retrieved from: https://www.researchgate.net/publication/245856177_Defusing_the_Confusion_Concepts_and_Measures_of_Continuity_of_Health_Care.
Lee J, Choi E, Choo E, Linda S, Jang EJ, Lee I-H. Relationship between continuity of care and clinical outcomes in patients with dyslipidemia in Korea: a real world claims database study. Sci Rep. 2022;12:3062. https://doi.org/10.1038/s41598-022-06973-3.
Pollack CE, Weissman GE, Lemke KW, Hussey PS, Weiner JP. Patient sharing among physicians and costs of care: a network analytic approach to care coordination using claims data. J Gen Intern Med. 2013;28:459–65. https://doi.org/10.1007/s11606-012-2104-7.
Bentler SE, Morgan RO, Virnig BA, Wolinsky FD. Do claims-based continuity of care measures reflect the patient perspective? Med Care Res Rev. 2014;71:156–73. https://doi.org/10.1177/1077558713505909.
Uijen AA, Heinst CW, Schellevis FG, van den Bosch WJHM, van de Laar FA, Terwee CB, Schers HJ. Measurement properties of questionnaires measuring continuity of care: a systematic review. PLoS ONE. 2012;7:e42256. https://doi.org/10.1371/journal.pone.0042256.
Nyweide DJ. Concordance between continuity of care reported by patients and measured from administrative data. Med Care Res Rev. 2014;71:138–55. https://doi.org/10.1177/1077558713505685.
Pazan F, Wehling M. Polypharmacy in older adults: a narrative review of definitions, epidemiology and consequences. Eur Geriatr Med. 2021. https://doi.org/10.1007/s41999-021-00479-3.
Cadogan CA, Ryan C, Hughes CM. Appropriate polypharmacy and medicine safety: when many is not too many. Drug Saf. 2016;39:109–16. https://doi.org/10.1007/s40264-015-0378-5.
Burt J, Elmore N, Campbell SM, Rodgers S, Avery AJ, Payne RA. Developing a measure of polypharmacy appropriateness in primary care: systematic review and expert consensus study. BMC Med. 2018;16:91. https://doi.org/10.1186/s12916-018-1078-7.
Sirois C, Simard M, Gosselin E, Gagnon M-E, Roux B, Laroche M-L. Mixed bag “polypharmacy”: methodological pitfalls and challenges of this exposure definition. Curr Epidemiol Rep. 2019;6:390–401. https://doi.org/10.1007/s40471-019-00214-4.
Choo E, Choi E, Lee J, Siachalinga L, Jang EJ, Lee I-H. Assessment of the effects of methodological choice in continuity of care research: a real-world example with dyslipidaemia cohort. BMJ Open. 2021;11:e053140. https://doi.org/10.1136/bmjopen-2021-053140.
Wastesson JW, Morin L, Tan ECK, Johnell K. An update on the clinical consequences of polypharmacy in older adults: a narrative review. Expert Opin Drug Saf. 2018;17:1185–96. https://doi.org/10.1080/14740338.2018.1546841.
Chen C-C, Tseng C-H, Cheng S-H. Continuity of care, medication adherence, and health care outcomes among patients with newly diagnosed type 2 diabetes: a longitudinal analysis. Med Care. 2013;51:231–7. https://doi.org/10.1097/MLR.0b013e31827da5b9.
Kerse N, Buetow S, Mainous AG, Young G, Coster G, Arroll B. Physician-patient relationship and medication compliance: a primary care investigation. Ann Fam Med. 2004;2:455–61. https://doi.org/10.1370/afm.139.
Robles S, Anderson GF. Continuity of care and its effect on prescription drug use among Medicare beneficiaries with hypertension. Med Care. 2011;49:516–21. https://doi.org/10.1097/MLR.0b013e31820fb10c.
Facchinetti G, D’Angelo D, Piredda M, Petitti T, Matarese M, Oliveti A, de Marinis MG. Continuity of care interventions for preventing hospital readmission of older people with chronic diseases: a meta-analysis. Int J Nurs Stud. 2020;101:103396. https://doi.org/10.1016/j.ijnurstu.2019.103396.
Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3:159–66. https://doi.org/10.1370/afm.285.
Adler R, Vasiliadis A, Bickell N. The relationship between continuity and patient satisfaction: a systematic review. Fam Pract. 2010;27:171–8. https://doi.org/10.1093/fampra/cmp099.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was funded by the German Innovation Fund, grant number 01VSF16034.
Conflicts of Interest
David Lampe, John Grosser, Daniel Gensorowsky, Julian Witte, Wolfgang Greiner, Christiane Muth, Marjan van den Akker, and Truc Sophia Dinh declare that they have no conflict of interest.
Availability of data and material
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
JW, DG, WG, CM, TSD, and MvdA contributed to the study conception and provided comments/revisions to the manuscript. DL and JG contributed to the screening and execution of the data extraction. DL and JG drafted the manuscript. JW, DG, WG, CM, TSD, and MvdA read and approved the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lampe, D., Grosser, J., Gensorowsky, D. et al. The Relationship of Continuity of Care, Polypharmacy and Medication Appropriateness: A Systematic Review of Observational Studies. Drugs Aging 40, 473–497 (2023). https://doi.org/10.1007/s40266-023-01022-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-023-01022-8