Abstract
Background
Treatment of schizophrenia requires long-term medication to prevent relapse. Treatment nonadherence may increase the risk of relapse, leading to increased hospitalizations and emergency room (ER) visits. Long-acting injectables (LAIs) such as paliperidone palmitate have improved treatment adherence and therefore symptoms. However, real-world studies comparing 3-monthly LAI formulations with other LAIs and oral antipsychotics (OAs) are scarce.
Objective
The objective of this study was to investigate and evaluate the clinical effectiveness of paliperidone palmitate LAI monthly (PP1M; Xeplion®) and 3-monthly (PP3M; Trevicta®) formulations compared with the monthly LAI aripiprazole (AM; Abilify Maintena®) and OAs in Spain.
Methods
This was a retrospective, observational study including 2275 adult patients with schizophrenia in a Spanish population. Data from hospital, primary care, and pharmacy dispensation electronic medical records were obtained between January 2017 and February 2018. The main outcomes included psychiatric hospitalizations and ER visit rates, days on treatment, and treatment persistence.
Results
Patients receiving PP3M had a significantly lower mean hospitalization rate (0.00046 ± standard deviation [SD] 0.00181; p < 0.0001) than other treatment groups. Kaplan–Meier curves revealed that 92.0 and 88.4% of patients receiving PP3M remained hospitalization free by 12 and 18 months, respectively. All treatment groups had at least a twofold significantly higher risk of psychiatric hospitalizations compared with those receiving PP3M or OAs, and the hospitalization risk among the PP3M group was significantly lower (hazard ratio [HR] 0.46; 95% confidence interval [CI] 0.31–0.67). The risk of ER visits was significantly lower with both PP3M and PP1M than with OAs, and lowest with PP3M (HR 0.462 [95% CI 0.29–0.62] and HR 0.833 [95% CI 0.59–0.97], respectively). Time until treatment switch with PP3M was high, with more than 86.5% of patients remaining on treatment at 18 months.
Conclusions
PP3M was more effective than OAs and monthly LAIs in improving clinical outcomes for patients with schizophrenia in a real-world setting in Spain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients receiving 3-monthly paliperidone palmitate had significantly lower mean rates of psychiatric hospitalization and emergency room visits than other treatment groups. |
In total, 92 and 88.4% of those receiving 3-monthly paliperidone palmitate remained hospitalization free at 12 and 18 months, respectively. |
The highest treatment persistence rates were observed with 3-monthly paliperidone palmitate, with 86.5% of these patients remaining on treatment by 18 months. |
1 Introduction
Schizophrenia is a chronic neuropsychiatric disorder affecting more than 21 million people globally [1]. The prevalence and incidence of schizophrenia are estimated to be 4.6 per 1000 and 15.2 per 100,000, respectively, with a median male:female rate ratio of 1.4:1 [2]. In Spain, real-world evidence suggests a greater prevalence (6.2 per 1000 people) and incidence rate (50.25 per 100,000 person-years) and a higher burden on healthcare resources than regional estimates [3]. Schizophrenia is further associated with lower average life expectancy and quality of life [4]. Relief from acute symptoms, prevention of relapse and hospitalizations, and treatment adherence are the key goals for long-term schizophrenia management [5, 6].
Continuous antipsychotic therapy has been recommended to avoid relapse [7]. High relapse rates [8] and decompensations because of poor medication adherence can affect long-term outcomes [9, 10]. Patient adherence to medication is important for controlling symptoms and relapse rates and preventing deterioration. Nonadherence to medication increased the risk of relapse 4.8 times and was the single biggest relapse predictor [11]. Real-world evidence from Germany showed that the use of long-acting injectables (LAIs) such as once-monthly aripiprazole (AM) reduced hospitalization rates, length of hospital stays, and psychotic episodes [12]. LAIs can be useful for improving patient adherence and preventing relapse [7]. Nonetheless, despite these advantages, their use remains low, with prescriptions of LAIs ranging between 7 and 22% of all antipsychotic prescriptions across different European countries [13].
Studies comparing the efficacy or effectiveness of different formulations of antipsychotics have found conflicting results. A meta-analysis of randomized controlled trials (RCTs) evaluating LAIs showed no benefit in preventing relapse or improving treatment adherence compared with oral antipsychotics (OAs) [14]. One possibility for this finding is that highly structured RCTs may not capture the benefit of treatment adherence observed in real-world clinical practice [15, 16], highlighting the importance of study design. A retrospective cohort analysis of patients with schizophrenia showed a lower risk of hospitalization with LAIs than with OAs [17]. A recent meta-analysis demonstrated fewer hospitalizations and emergency room (ER) visits with monthly LAIs than with OAs [18]. Similarly, patients receiving monthly formulations of aripiprazole were previously shown to have longer time to discontinuation than those receiving OAs [19], suggesting a benefit from the monthly formulations of LAIs for long-term management of schizophrenia. However, very few real-world effectiveness studies have compared the benefits of once-monthly and 3-monthly LAIs.
New LAIs, such as paliperidone palmitate, may improve and promote patient adherence and therefore improve symptom control [20, 21]. To date, paliperidone palmitate is the only LAI with a 3-monthly formulation (PP3M), and this formulation has a good safety profile and significantly delays the time to relapse in patients with schizophrenia [22]. A retrospective longitudinal study in patients with schizophrenia receiving PP3M reported reduced use of OAs, time spent in inpatient settings, number of outpatient visits, and associated healthcare costs [23]. Another study, with a naturalistic follow-up of outpatients treated with PP3M for 2 years, found PP3M to be highly effective in relapse prevention and treatment discontinuation in patients with schizophrenia [24]. Further, PP3M was noninferior to once-monthly paliperidone palmitate (PP1M) in terms of safety and tolerability profile, pharmacokinetics, and relapse rates in patients with schizophrenia, indicating its potential for use in patients experiencing difficulty with treatment adherence and relapses [25]. Studies evaluating the cost effectiveness of PP3M compared with PP1M showed fewer relapses and reduced hospitalizations and ER visits in both Spanish [26] and Dutch populations [27]. Reduced hospitalizations and ER visits may indicate improved symptomatic control and adherence to paliperidone palmitate treatments in real-world clinical settings. However, more studies are needed to evaluate the real-world effectiveness of monthly and 3-monthly LAIs compared directly with OAs available in Spain.
The aim of this study was to investigate and evaluate the clinical effectiveness of PP1M and PP3M compared with LAI AM and OAs in Spain, by comparing rates of psychiatric hospitalization and ER visits and treatment persistence. The specific hypothesis was that prolonged 3-monthly formulations such as PP3M would improve treatment adherence compared with monthly LAIs and OAs, reducing the risk of hospitalization and ER visits and increasing treatment persistence.
2 Methods
2.1 Study Design and Data Source
We conducted a noninterventional, observational, retrospective study using IQVIA’s population-based electronic medical records (EMRs) database, which includes hospital, primary care, and pharmacy dispensation EMRs of approximately 1.8 million inhabitants from four regions in Spain. Identifying data were deleted from the database records in accordance with data protection laws.
Data extracted from the EMR database between January 2017 and December 2018 included patient characteristics (age, sex), diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM], the Spanish version of the ICD-10 [ICD-10-ES], and the International Classification of Primary Care [ICPC-2]), disease-related comorbidities and other significant diseases, drug dispensing information (dispensation dates, anatomical therapeutic chemical class, substance name, dose, treatment duration, and administration route), and reasons for hospitalization. The Spanish coding system for the minimum basic data set is based on the ICD-9-CM and the ICD-10-ES. Although the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition is available for use in mental health, both systems are multiaxial and used to classify patients with these types of conditions. The number of hospitalizations during 2016 was available for all patients.
2.2 Study Population
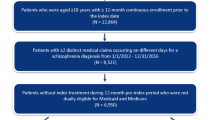
Inclusion criteria were (1) adults aged ≥ 18 years with (2) a schizophrenia diagnosis based on ICD-9-CM/ICD-10-ES (schizophrenia disorder codes: 295/F20) and ICPC-2 (schizophrenia code: P72) (3) undergoing treatment with any of PP3M, PP1M, AM, or OA (including oral drugs listed in S6 (including oral drugs listed in the electronic supplementary material [ESM], S6, approved in Spain), approved in Spain) and (4) with prescriptions for the start of a new treatment period (treatment initiation). In the case of this fourth assumption, for patients treated with PP1M, AM, or OA, we considered only patients who started treatment in March 2017 so as to exclude patients with treatment established before the study initiation. Patients who started treatment with PP3M after January 2017 were considered. This consideration regarding treatment initiation was necessary because treatment information was taken from the dispensation records, and dispensation data were not available before 2017. The following two assumptions were made when considering treatment initiation. First, because PP3M was marketed in Spain in October 2016 and is a 3-monthly formulation, all patients receiving PP3M from January 2017 were considered for the analysis. Second, dispensations of PP1M, AM, and OAs are usually monthly; however, because the number of days between treatment dispensations may vary slightly, we required a broader timeframe to ensure that these were a treatment initiation. For this reason, we included only patients whose first dispensation was from March 2017.
Exclusion criteria was receipt of clozapine treatment (N = 298) since it is only indicated for the treatment of treatment-resistant schizophrenia. Other LAIs were also excluded from the study because the low number of patients meant the data were unsuitable for comparisons (zuclopenthixol, n = 19; fluphenazine, n = 28; risperidone, n = 10).
Patients included in the study were likely to undergo treatment switches during the observation period. Because of this, we undertook an as-treated analysis, where patients counted toward each treatment period under a specific drug treatment, to account for all treatments received during the observation period [28]. As such, two sample sizes were used for this study: total number of patients and total number of treatments (see the electronic supplementary material [ESM], S1).
The flow chart in ESM 2 demonstrates the selection of study participants.
2.3 Outcomes
All outcome measures were assessed after the index date. The outcome measures were as follows: number of psychiatric hospitalizations (defined as all psychiatric hospitalizations during the observation period), number of ER visits (defined as all ER visits during the observation period), treatment time (defined as the duration in days of each specific continuous treatment), psychiatric hospitalization rate (defined as the number of psychiatric hospitalizations occurring within one treatment/total continuous treatment time under that treatment), ER rate (defined as the number of ER visits occurring within one treatment/total continuous treatment time under that treatment), and treatment persistence and switches to other treatments (defined as the non-discontinuation of the ongoing treatment and switching when the drug is discontinued and another treatment is prescribed). In terms of treatment switches, for patients who switched multiple times, switching was considered consecutively, between each previous treatment and the following one (e.g., PP3M–PP1M–OA: Switch counts for PP3M to PP1M and PP1M to OA). The occurrence of each event counted toward the ongoing treatment at the time the event was experienced.
2.4 Subgroup Analysis by Age
Patients were stratified by age (≤ 40 and > 40 years) to evaluate differences in clinical outcomes among younger and older patients both between treatment groups and among the same treatment group.
2.5 Statistical Analysis
We generated summary statistics for baseline characteristics, with continuous variables described using the number of patients with valid/missing observations, mean, standard deviation (SD), median, 25th and 75th percentiles, interquartile range (IQR), and minimum and maximum, and categorical variables described using frequencies and related percentages. We used one-way analysis of variance (ANOVA) for statistical comparisons and used p-value-adjusted pairwise comparisons using the Tukey method. We used analysis of covariance (ANCOVA) to control for potential confounders and included adjusting by variables that were identified as statistically significant among groups in the ANOVA analysis (age and previous psychiatric hospitalization in 2016). We tested the correlation among variables to avoid collinearity, using Pearson’s correlation methods and following Cohen’s rule of thumb for interpretation. When collinearity existed, we used the most relevant variable.
We used Kaplan–Meier methods to analyze the time-to-event probabilities and compare survival between treatment groups. Events were considered separately as time to hospitalization, time to ER visit, or time until treatment switch. Censuring occurred when patients were lost to follow-up or when observation time occurred before the occurrence of any event. We used the Šidák and Log-rank tests to assess the statistical significance of differences among groups and estimated hazard ratios (HRs) and their respective confidence intervals (CIs) using a Cox proportional hazards regression model among pairwise treatments to compare the risk of having an event using PP3M as reference. HRs were also computed with OAs and AM as reference. The study sample was sufficient to detect 8% difference between groups in terms of hospitalization risk, with an α error of 0.05 and a β error of 0.2. Statistical analyses were performed using SAS® 9.3, and p-values less than 0.05 were considered statistically significant.
3 Results
A total of 5321 patients with schizophrenia were identified during the study period, of whom 2275 were eligible (ESM S1 and S2). During the treatment period, each patient counted toward other treatment categories when the main treatment changes occurred during the observation period, leading to a total of 3188 total person-treatments (ESM S1.B). Therefore, during the study period, a total of 3188 treatments took place among the 2275 patients included.
3.1 Baseline Characteristics
Table 1 shows the baseline demographic and clinical characteristics of patients according to their first treatment. The mean age was 46.8 years, and patients were predominantly male (62.9%). Differences at baseline were observed regarding age, with a higher proportion of younger patients in the AM group and a higher proportion of males among the PP1M and PP3M groups. Psychiatric hospitalizations in 2016 were highest in the OA group, whereas non-psychiatric hospitalizations in 2016 were highest among the AM group.
We conducted one-way ANOVA followed by Tukey’s post hoc analysis to identify significant differences among groups in age, number of comorbidities, time since diagnosis, and previous psychiatric hospitalizations in 2016 (Table 2). A significant correlation between age and time since diagnosis was observed, for which age was preferred over time since diagnosis. Statistically significant differences across groups were identified for age, with the AM group having a lower age than the other groups (mean 39.92 years), and for previous psychiatric hospitalizations in 2016, with PP3M having the least number of mean previous hospitalizations (0.16) and AM (0.66) having the highest.
3.2 Psychiatric Hospitalizations
Although the mean number of psychiatric hospitalizations differed among groups, no differences were observed after ANCOVA adjustment by age and previous psychiatric hospitalizations in 2016 (Table 3). The most frequent reason for psychiatric hospitalization was schizophrenia itself, ranging between 64.4 for OA and 78.0% for PP1M, followed by depressive disorders. We also analyzed hospitalization rates, accounting for hospitalization events over time spent on treatment. Patients receiving PP3M had the lowest mean hospitalization rate. After adjusting for confounders, statistically significant differences remained for PP3M (0.0006 hospitalization treatment-days) and OA (0.0015 hospitalization treatment-days) (p = 0.0006) (Table 3).
PP3M was associated with a significant delay until psychiatric hospitalization events (Fig. 1). At 12 months, 92% of patients receiving PP3M remained hospitalization free. At 18 months, 88.4% of patients receiving PP3M remained hospitalization free (95% CI 85.0–90.4), followed by those receiving PP1M (72.1%; 95% CI 66.2–77.1), AM, and OAs. All treatment groups had at least a twofold significantly higher risk of psychiatric hospitalization than those receiving PP3M (Table 4). Additionally, only the PP3M group had a significantly lower risk of hospitalization when OA was the reference (HR 0.456; 95% CI 0.31–0.67).
3.3 Emergency Room Visits
Only the rate of ER visits displayed statistically significant differences across some groups after adjustment, with PP3M and PP1M groups differing significantly from those receiving OAs (Table 3). Time until ER visit among the PP3M group was significantly delayed compared with other treatments, with 64.2% (95% CI 60.0–68.0) remaining ER visit free after 18 months, followed by the PP1M group (46.8%; 95% CI 40.6–52.8) and those receiving OAs (41.7%; 95% CI 38.1–45.4) or AM (36.5%; 95% CI 26.4–46.6) (Fig. 2). All treatment groups had a significantly higher risk of ER visit compared with PP3M, ranging from an HR of 1.84 for PP1M to an HR of 2.17 for OAs. The risk of ER visits was significantly lower with both PP3M and PP1M than with OAs (HR 0.462 [95% CI 0.29–0.62] and HR 0.833 [95% CI 0.59–0.97], respectively) (Table 4). Furthermore, when both once-monthly LAIs were compared, the PP1M group had a 25% lower risk of ER visits than those receiving AM.
3.4 Time Until Treatment Switch
Treatment time varied across groups; however, after adjustments for age and previous psychiatric hospitalizations in 2016, statistically significant differences remained only between the PP3M and other OA groups (adjusted means: PP3M 472.09 days, OA 249.28 days; Table 3). The median dose for each treatment over the study period was 350 mg (IQR 263.0–525.0) for PP3M, 100 mg (IQR 75.7–136.7) for PP1M, and 400 mg (IQR 312.5–400.0) for AM. Treatment persistence, in terms of continuing the same treatment during follow-up, was highest for PP3M, with 86.5% (95% CI 83.4–89.0) of patients continuing after 18 months (Fig. 3). As expected, time until treatment switch was lowest for PP1M (43.5% at 18 months; 95% CI 41.3–45.9) since most patients transitioned into the 3-monthly formulation (90.8% of all PP1M treatment switches were to PP3M). All treatment groups had a more than threefold significantly higher risk than the PP3M group of switching treatments. The risk of switching treatment was significantly lower for both PP3M and AM when OA was used as the reference: HR 0.29 (95% CI 0.15–0.62) and HR 0.64 (95% CI 0.53–0.79), respectively (Table 4). Comparisons between once-monthly LAIs showed that the risk of switching from PP1M to other treatments (not including PP3M) was 1.57 times higher than with AM (95% CI 1.52–1.59).
3.5 Age Subgroup Analysis
When patients were stratified by age for treatment comparisons, that those receiving PP3M had a lower risk of psychiatric hospitalization and ER visits and greater treatment persistence, irrespective of age. However, for treatment persistence, differences were more notable among those aged > 40 years (ESM S3 and S5).
For intratreatment group comparisons, patients aged > 40 years had a lower risk of hospitalization than those aged < 40 years with both PP1M and OA, whereas differences for PP3M and AM were nonsignificant. In terms of ER visits, only PP1M showed statistically significant differences among age groups, with a higher risk of ER visits among patients aged ≤ 40 years than among those aged > 40 years (ESM S4). Treatment persistence differed by age for OA and PP1M.
4 Discussion
To date, evidence on the clinical effectiveness of LAIs compared with OAs has been under-researched, and results have remained conflicting. Furthermore, real-world evidence on 3-monthly LAIs is scarce. In the present study, we report several advantages observed for clinical outcomes among patients receiving PP3M, especially when compared with OAs. Those receiving PP3M had lower hospitalization rates than those on other treatments, and 88.4% of patients remained hospitalization free by 18 months. Additionally, all treatment groups had an at least twofold significantly higher risk of psychiatric hospitalization than those receiving PP3M. Similarly, PP3M-treated patients had the lowest ER visit rates. Treatment time was longest among the PP3M group compared with other groups. Time until treatment switch was high, with over 90% of patients continuing PP3M treatment by 12 months, whereas those receiving other treatments were three times more likely to change treatment over time.
Relapses and decompensations of acute psychotic symptoms may lead to increased psychiatric hospitalizations [29]. PP1M has been shown to reduce the number of hospitalizations and the duration of hospital stays [30]. A pre–post analysis of patients receiving PP1M reported reductions in hospital admissions and length of stay after 3 years of 63 and 220%, respectively [31]. Additionally, a study in patients with schizophrenia following at least 1 year of treatment observed lower hospital admissions among patients treated with PP3M than among those receiving AM [32]. Our findings are in line with these observations, although we highlight that 3-monthly formulations yielded further improvements by significantly reducing the psychiatric hospitalization rates compared with the other treatments. The mean hospitalization rates for patients treated with PP3M were lowest (0.00046), whereas the highest hospitalization rates (0.00146)—nearly three times higher than with PP3M—were seen with OAs. Our findings further support results from a recent systematic review and meta-analysis of data arising from RCTs, cohort studies, and pre–post studies, where LAIs decreased hospitalization and relapse compared with OAs [33]. Reductions in hospitalization suggest that LAI-treated patients are likely to experience good symptom control, leading to fewer relapses and decompensations, which could improve long-term prognosis and patient quality of life.
The prevalence of psychiatric disorders is high among patients with frequent ER visits (more than one per year). Among those with recurrent ER visits (more than four per year), schizophrenia-related visits were the third most frequent reason for recurrence [34]. In our study, by the end of the study period, all patients receiving paliperidone palmitate displayed a lower risk of ER visits compared with other treatments, although the effect was more marked for those receiving PP3M. Although we did not analyze the underlying cause of ER visits, lower rates of ER visits are relevant in terms of improving ER overcrowding, which has a significant financial impact on the healthcare system [35, 36]. In turn, these findings suggest that a favorable economic impact could be expected from patients treated with PP3M, based on the reduced hospitalizations and ER visits, although reduced hospitalizations and length of stay are likely to play a greater role in terms of costs.
PP3M was associated with a longer treatment time and lower likelihood of switching treatments. Our findings are in line with earlier studies reporting improved patient adherence with PP3M [20, 21, 37], which may lead to significant symptomatic and functional remission and decreased hospitalization [38]. Additionally, the high percentage of patients on PP1M transitioning to PP3M (90.8% of all PP1M switches at 18 months were to PP3M), together with the high proportion of patients persisting with treatment, even by 18 months, highlights the likely good tolerability and effectiveness of both paliperidone palmitate formulations, with PP3M facilitating patient management. Our results are further supported by a recent study in which patients stabilized on PP1M and switched to PP3M had fewer rehospitalizations than with previous treatments [39].
Studies comparing the effectiveness of LAIs are scarce. In our study, PP3M outperformed both monthly LAIs (PP1M and AM) in time to psychiatric hospitalization, ER visits, and time to treatment switches. A study analyzing the risk of relapse with different paliperidone palmitate formulations concluded that relapse rates were progressively lower with longer-acting formulations [40]. One possible explanation for the reduced effectiveness of shorter-acting paliperidone palmitate formulations could be the direct associations between treatment compliance and PP1M effectiveness, as reported by some studies [41]. When we compared the two monthly LAI formulations, we were unable to identify differences among PP1M and AM in terms of hospitalization risk, whereas we identified a lower risk of ER visits among the PP1M group and a lower risk of switching treatment for AM compared with PP1M (however, low PP1M treatment persistence reflects a high transition from PP1M to PP3M, which represented 90.8% of PP1M switches at 18 months). A study reported similar hospitalization rates among those receiving PP1M or other LAIs after 3 years of treatment, despite the PP1M group having more hospitalizations at baseline, suggesting more severe disease [20]. In this sense, within our study, patients receiving PP1M and PP3M were older than those receiving AM, suggesting that clinicians may be inclined to prescribe PP1M or PP3M in patients with a longer disease course. However, a recent study reported that PP3M is effective in achieving symptomatic remission both in younger patients (age < 35 years) and in patients newly diagnosed with schizophrenia (< 3 years) [42]. Further studies are needed to confirm our findings.
Information on the effectiveness of LAIs according to age is sparse. Our findings seem to be independent of age (all results were age adjusted, and differences among age intratreatment groups were limited), in line with previous findings [43].
4.1 Limitations
This real-world study benefited from a large and population-based representative sample and a long follow-up period and reflects real clinical practice in Spain. However, some limitations and considerations must be acknowledged. This study relied on administrative data, which is subject to coding errors and missing data, and diagnoses are entered for administrative processing purposes rather than for clinical completeness, all of which could reduce the validity of our conclusions. Drug treatment information was based on pharmacy dispensation, but dispensation and treatment adherence could differ. Clozapine-treated patients were excluded from this study. In turn, this study does not describe treatment-resistant schizophrenia. Additionally, we excluded other LAIs from the analyses because the small sample sizes made comparisons difficult. Only outpatient dispensation was analyzed. However, data on inpatient use of antipsychotics were not available, which could affect the outcomes considered here, although this should have little impact on our findings. Duration of illness has been associated with treatment response [44]. However, because of the correlation with age, we were unable to include this in our analyses. Prior hospitalizations were only available for 2016, and the analysis could have benefited from a longer pre-treatment observation period. The database relied on pharmaceutical dispensation, so we could not assess monotherapy and polytherapy, particularly because of difficulties in defining a minimum period of consecutive purchases to consider polytherapy treatment, as well as difficulties grouping OAs by anatomical therapeutic chemical class. Lastly, when interpreting our results, it should be remembered that PP3M is usually given to patients already stabilized on PP1M. In turn, it could be argued that low relapse and discontinuation rates should be expected.
5 Conclusions
This study suggests that PP3M was more effective than OAs and PP1M and AM at improving clinical outcomes for patients with schizophrenia in a real-world setting in Spain. Patients receiving PP3M showed sustained treatment adherence and reduced hospitalization and ER visits. Longer-acting formulations have the potential to further improve the disease course.
References
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76.
Orrico-Sánchez A, López-Lacort M, Munõz-Quiles C, Sanfélix-Gimeno G, Diéz-Domingo J. Epidemiology of schizophrenia and its management over 8-years period using real-world data in Spain. BMC Psychiatry. 2020;20:149.
Toudic C, Berrouiguet S, Lemey C, Walter M. Life expectancy, quality of life and schizophrenia. French J Psychiatry. 2019;1:S127.
Galderisi S, Färden A, Kaiser S. Dissecting negative symptoms of schizophrenia: history, assessment, pathophysiological mechanisms and treatment. Schizophr Res. 2017;186:1–2.
Majer IM, Gaughran F, Sapin C, Beillat M, Treur M. Efficacy, tolerability, and safety of aripiprazole once-monthly versus other long-acting injectable antipsychotic therapies in the maintenance treatment of schizophrenia: a mixed treatment comparison of double-blind randomized clinical trials. J Mark Access Health Policy. 2015;3:27208.
Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14:2–44.
Emsley R, Chiliza B, Asmal L, Harvey BH. The nature of relapse in schizophrenia. BMC Psychiatry. 2013;13:50.
Spaniel F, Bakstein E, Anyz J, Hlinka J, Sieger T, Hrdlicka J, et al. Relapse in schizophrenia: definitively not a bolt from the blue. Neurosci Lett. 2018;669:68–74.
Xiao J, Mi W, Li L, Shi Y, Zhang H. High relapse rate and poor medication adherence in the chinese population with schizophrenia: results from an observational survey in the people’s Republic of China. Neuropsychiatr Dis Treat. 2015;11:1161–7.
Caseiro O, Pérez-Iglesias R, Mata I, Martínez-Garcia O, Pelayo-Terán JM, Tabares-Seisdedos R, et al. Predicting relapse after a first episode of non-affective psychosis: a three-year follow-up study. J Psychiatr Res. 2012;46:1099–105.
Thiem H, Folkerts H, Völkel L. Short-acting antipsychotics or long-acting injectables? A treatment comparison in patients with schizophrenia. Gesundheitsokonomie und Qual. 2020;25:170–8.
Arango C, Baeza I, Bernardo M, Cañas F, de Dios C, Díaz-Marsá M, et al. Long-acting injectable antipsychotics for the treatment of schizophrenia in Spain. Ed E, editor. Rev Psiquiatr Salud Ment. 2019;12:92–105.
Kishimoto T, Robenzadeh A, Leucht C, Leucht S, Watanabe K, Mimura M, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40:192–213.
Kirson NY, Weiden PJ, Yermakov S, Huang W, Samuelson T, Offord SJ, et al. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry. 2013;74:568–75.
Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74:957–65.
MacEwan JP, Kamat SA, Duffy RA, Seabury S, Chou JW, Legacy SN, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67:1183–8.
Lin D, Thompson-Leduc P, Ghelerter I, Nguyen H, Lafeuille MH, Benson C, et al. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35:469–81.
Yan T, Greene M, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of aripiprazole once-monthly 400 mg (AOM 400) versus oral antipsychotics in patients with schizophrenia or bipolar I disorder: a real-world study using US claims data. Adv Ther. 2018;35:1612–25.
Patel R, Chesney E, Taylor M, Taylor D, McGuire P. Is paliperidone palmitate more effective than other long-acting injectable antipsychotics? Psychol Med. 2018;48:1616–23.
Pilon D, Muser E, Lefebvre P, Kamstra R, Emond B, Joshi K. Adherence, healthcare resource utilization and Medicaid spending associated with once-monthly paliperidone palmitate versus oral atypical antipsychotic treatment among adults recently diagnosed with schizophrenia. BMC Psychiatry. 2017;17:207.
Berwaerts J, Liu Y, Gopal S, Nuamah I, Xu H, Savitz A, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia a randomized clinical trial. JAMA Psychiat. 2015;72:830–9.
DerSarkissian M, Lefebvre P, Joshi K, Brown B, Lafeuille MH, Bhak RH, et al. Health care resource utilization and costs associated with transitioning to 3-month paliperidone palmitate among US veterans. Clin Ther. 2018;40:1496–508.
Clark I, Wallman P, Cornelius V, Taylor D. Factors predicting relapse and treatment discontinuation with paliperidone 3-monthly long-acting injection: a 2-year naturalistic follow-up study. Eur Psychiatry. 2021;64:68.
Savitz AJ, Xu H, Gopal S, Nuamah I, Ravenstijn P, Janik A, et al. Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharmacol. 2016;19:pyw018.
Einarson TR, Bereza BG, Garcia Llinares I, González Martín Moro B, Tedouri F, Van Impe K. Cost-effectiveness of 3-month paliperidone treatment for chronic schizophrenia in Spain. J Med Econ. 2017;20:1039–47.
Einarson TR, Bereza BG, Tedouri F, Van Impe K, Denee TR, Dries PJT. Cost-effectiveness of 3-month paliperidone therapy for chronic schizophrenia in the Netherlands. J Med Econ. 2017;20:1187–99.
Danaei G, Rodríguez LAG, Cantero OF, Logan R, Hernán MA. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22:70–96.
Almond S, Knapp M, Francois C, Toumi M, Brugha T. Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry. 2004;184:346–51.
Nikolić N, Page N, Akram A, Khan M. The impact of paliperidone palmitate long-acting injection on hospital admissions in a mental health setting. Int Clin Psychopharmacol. 2017;32:95–102.
Pappa S, Mason K, Howard E. Long-term effects of paliperidone palmitate on hospital stay and treatment continuation. Int Clin Psychopharmacol. 2019;34:305–11.
Garciá-Carmona JA, Simal-Aguado J, Campos-Navarro MP, Valdivia-Muñoz F, Galindo-Tovar A. Evaluation of long-acting injectable antipsychotics with the corresponding oral formulation in a cohort of patients with schizophrenia: a real-world study in Spain. Int Clin Psychopharmacol. 2020;36:18–24.
Kishimoto T, Hagi K, Kurokawa S, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre–post studies. Lancet Psychiatry. 2021;8:387–404.
Slankamenac K, Heidelberger R, Keller DI. Prediction of recurrent emergency department visits in patients with mental disorders. Front Psychiatry. 2020;11:48.
Foley M, Kifaieh N, Mallon WK. Financial impact of emergency department crowding. West J Emerg Med. 2011;12:192–7.
Richardson LD, Asplin BR, Lowe RA. Emergency department crowding as a health policy issue: past development, future directions. Ann Emerg Med. 2002;40:388–93.
Schreiner A, Aadamsoo K, Altamura AC, Franco M, Gorwood P, Neznanov NG, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169:393–9.
Garcia-Portilla MP, Llorca P-M, Maina G, Bozikas VP, Devrimci-Ozguven H, Kim S-W, et al. Symptomatic and functional outcomes after treatment with paliperidone palmitate 3-month formulation for 52 weeks in patients with clinically stable schizophrenia. Ther Adv Psychopharmacol. 2020;10:204512532092634.
Wallman P, Clark I, Taylor D. Effect of 3-monthly paliperidone palmitate on hospitalisation in a naturalistic schizophrenia cohort—a five-year mirror image study. J Psychiatr Res. 2022;148:131–6.
Mathews M, Gopal S, Singh A, Nuamah I, Pungor K, Tan W, et al. Comparison of relapse prevention with 3 different paliperidone formulations in patients with schizophrenia continuing versus discontinuing active antipsychotic treatment: a post-hoc analysis of 3 similarly designed randomized studies. Neuropsychiatr Dis Treat. 2020;16:1533–42.
Laing E, Taylor D. Relapse and frequency of injection of monthly paliperidone palmitate—a retrospective case–control study. Eur Psychiatry. 2021;64:11.
Pungor K, Bozikas VP, Emsley R, Llorca P-M, Gopal S, Mathews M, et al. Stable patients with schizophrenia switched to paliperidone palmitate 3-monthly formulation in a naturalistic setting: impact of patient age and disease duration on outcomes. Ther Adv Psychopharmacol. 2020;10:204512532098150.
Targum SD, Risinger R, Du Y, Pendergrass JC, Jamal HH, Silverman BL. Effect of patient age on treatment response in a study of the acute exacerbation of psychosis in schizophrenia. Schizophr Res. 2017;179:64–9.
Altamura AC, Serati M, Buoli M. Is duration of illness really influencing outcome in major psychoses? Nord J Psychiatry. 2015;69:1685–99.
Acknowledgements
IQVIA assisted in drafting the manuscript under the direction of the authors and provided editorial support throughout its development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Janssen. Medical writing and editorial support were funded by Janssen. Open access publication was funded by Janssen.
Conflict of interest
LGR has been a speaker and advisory board member for Janssen-Cilag, Angelini, Casen-Recordati, Lundbeck, Otsuka, Novartis, GSK, and Pfizer. SSA has been a speaker and advisory board member for Janssen-Cilag, Angelini, Casen-Recordati, Lundbeck, Otsuka, Qualigen, and Adamed. MGD and PLR are full-time employees of Janssen.
Ethics approval
This study was conducted according to guidelines on observational post-authorization studies for medicinal products for human use specified in Orden SAS/3470/2009 of the Spanish Agency of Medicines and Medical Devices (AEMPS). The study was also conducted according to good clinical practice guidelines (International Conference for Harmonization) and the Declaration of Helsinki and was approved by a public hospital ethics committee in Spain.
Consent to participate
All identifiable personal information was removed for privacy protection prior to data acquisition, so the requirement for informed consent was waived.
Consent for publication
Not applicable.
Availability of data and materials
The data analyzed in this study are not publicly available according to the licensing agreement with IQVIA.
Code availability
Not applicable.
Author contributions
MGD and PLR designed the study concept. LGR, SSA, MGD, and PLR participated in data analysis and interpretation and in the review and editing of the article. LGR and SSA wrote the first draft. All authors critically reviewed the manuscript. All authors provided input into the drafting of the manuscript, have read, and approved the final submitted version to be published, and agree to be accountable for the work as a whole.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gutiérrez‐Rojas, L., Sánchez-Alonso, S., García Dorado, M. et al. Impact of 3-Monthly Long-Acting Injectable Paliperidone Palmitate in Schizophrenia: A Retrospective, Real-World Analysis of Population-Based Health Records in Spain. CNS Drugs 36, 517–527 (2022). https://doi.org/10.1007/s40263-022-00917-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-022-00917-1