Abstract
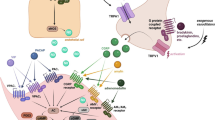
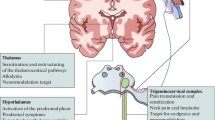
Migraine is a strongly disabling disease characterized by a unilateral throbbing headache lasting for up to 72 h for each individual attack. There have been many theories on the pathophysiology of migraine throughout the years. Currently, the neurovascular theory dominates, suggesting clear involvement of the trigeminovascular system. The most recent data show that a migraine attack most likely originates in the hypothalamus and activates the trigeminal nucleus caudalis (TNC). Although the mechanisms are unknown, activation of the TNC leads to peripheral release of calcitonin gene-related protein (CGRP), most likely from C-fibers. During the past year monoclonal antibodies against CGRP or the CGRP receptor have emerged as the most promising targets for migraine therapy, and at the same time established the strong involvement of CGRP in the pathophysiology of migraine. The viewpoint presented here focuses further on the activation of the CGRP receptor on the sensory Aδ-fiber, leading to the sensation of pain. The CGRP receptor activates adenylate cyclase, which leads to an increase in cyclic adenosine monophosphate (cAMP). We hypothesize that cAMP activates the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, triggering an action potential sensed as pain. The mechanisms behind migraine pain on a molecular level, particularly their importance to cAMP, provide clues to potential new anti-migraine targets. In this article we focus on the development of targets related to the CGRP system, and further include novel targets such as the pituitary adenylate cyclase-activating peptide (PACAP) system, the serotonin 5-HT1F receptor, purinergic receptors, HCN channels, adenosine triphosphate-sensitive potassium channels (KATP), and the glutaminergic system.


Similar content being viewed by others
References
Headache Classification Committee of the International Headache Society (IHS). The International classification of headache disorders, 3rd edition. Cephalalgia. 2018;38:1–211.
Tfelt-Hansen PC, Koehler PJ. One hundred years of migraine research: major clinical and scientific observations from 1910 to 2010. Headache. 2011;51:752–78.
Charles A. The evolution of a migraine attack—a review of recent evidence. Headache. 2013;53:413–9.
Schoonman GG, Evers DJ, Terwindt GM, van Dijk JG, Ferrari MD. The prevalence of premonitory symptoms in migraine: a questionnaire study in 461 patients. Cephalalgia. 2006;26:1209–13.
Quintela E, Castillo J, Munoz P, Pascual J. Premonitory and resolution symptoms in migraine: a prospective study in 100 unselected patients. Cephalalgia. 2006;26:1051–60.
Hougaard A, Amin FM, Hauge AW, Ashina M, Olesen J. Provocation of migraine with aura using natural trigger factors. Neurology. 2013;80:428–31.
Karsan N, Goadsby PJ. Biological insights from the premonitory symptoms of migraine. Nat Rev Neurol. 2018;14:699–710.
Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci. 2011;31:1937–43.
Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–60.
Schulte LH, Allers A, May A. Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology. 2017;88:2011–6.
Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016;139:1987–93.
Raffaelli E Jr, Menon AD. Migraine and the limbic system. Headache. 1975;15:69–78.
Rajmohan V, Mohandas E. The limbic system. Indian J Psychiatry. 2007;49:132–9.
Benedetti F, Carlino E, Pollo A. How placebos change the patient’s brain. Neuropsychopharmacology. 2011;36:339–54.
Lundblad C, Haanes KA, Grande G, Edvinsson L. Experimental inflammation following dural application of complete Freund’s adjuvant or inflammatory soup does not alter brain and trigeminal microvascular passage. J Headache Pain. 2015;16:91.
Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015;1600:93–109.
Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–6.
Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7.
Edvinsson L, Nilsson E, Jansen-Olesen I. Inhibitory effect of BIBN4096BS, CGRP(8-37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol. 2007;150:633–40.
Gupta S, Akerman S, van den Maagdenberg AM, Saxena PR, Goadsby PJ, van den Brink AM. Intravital microscopy on a closed cranial window in mice: a model to study trigeminovascular mechanisms involved in migraine. Cephalalgia. 2006;26:1294–303.
Amrutkar DV, Ploug KB, Hay-Schmidt A, Porreca F, Olesen J, Jansen-Olesen I. mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. Pain. 2012;153:830–8.
Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain. 2013;14:1289–303.
Gupta S, Mehrotra S, Avezaat CJ, Villalon CM, Saxena PR, MaassenVanDenBrink A. Characterisation of CGRP receptors in the human isolated middle meningeal artery. Life Sci. 2006;79:265–71.
Khan S, Amin FM, Christensen CE, Ghanizada H, Younis S, Olinger ACR, et al. Meningeal contribution to migraine pain: a magnetic resonance angiography study. Brain. 2019;142:93–102.
Ottosson A, Edvinsson L. Release of histamine from dural mast cells by substance P and calcitonin gene-related peptide. Cephalalgia. 1997;17:166–74.
Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, et al. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–23.
Melo-Carrillo A, Strassman AM, Nir RR, Schain AJ, Noseda R, Stratton J, et al. Fremanezumab-a humanized monoclonal anti-CGRP antibody-inhibits thinly myelinated (Aδ) but not unmyelinated (C) meningeal nociceptors. J Neurosci. 2017;37:10587–96.
Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–36.
Hansen JM, Sitarz J, Birk S, Rahmann AM, Oturai PS, Fahrenkrug J, et al. Vasoactive intestinal polypeptide evokes only a minimal headache in healthy volunteers. Cephalalgia. 2006;26:992–1003.
Hurley JH, Kunkler PE, Zhang L, Knopp KL, Oxford GS. Role of intraganglionic transmission in the trigeminovascular pathway. Mol Pain. 2019;15:1744806919836570.
Walker CS, Raddant AC, Woolley MJ, Russo AF, Hay DL. CGRP receptor antagonist activity of olcegepant depends on the signalling pathway measured. Cephalalgia. 2018;38:437–51.
Ashina H, Schytz HW, Ashina M (2018) CGRP in Human Models of Migraine. In: Handbook of experimental pharmacology. Springer, Berlin. https://doi.org/10.1007/164_2018_128
Brennan KC, Charles A. An update on the blood vessel in migraine. Curr Opin Neurol. 2010;23:266–74.
Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, et al. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–45.
Guo S, Vollesen AL, Olesen J, Ashina M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain. 2016;157:2773–81.
Guo S, Olesen J, Ashina M. Phosphodiesterase 3 inhibitor cilostazol induces migraine-like attacks via cyclic AMP increase. Brain. 2014;137:2951–9.
Birk S, Kruuse C, Petersen KA, Tfelt-Hansen P, Olesen J. The headache-inducing effect of cilostazol in human volunteers. Cephalalgia. 2006;26:1304–9.
Kruuse C, Thomsen LL, Birk S, Olesen J. Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain. 2003;126:241–7.
Ashina M (2018) Human models of migraine - short-term pain for long-term gain. MTIS London, Migraine Trust Lecture, 7th September.
Danish Headache Center. Headache inducing effect of cromakalim in migraine patients [ClinicalTrials.gov identifier NCT03228355]. National Institutes of Health, ClinicalTrials.gov. 2019. https://clinicaltrials.gov. Accessed 2 Apr 2019.
Liu Y, Shakur Y, Yoshitake M, Kambayashi JJ. Cilostazol (pletal): a dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc Drug Rev. 2001;19:369–86.
Haanes KA, Labastida-Ramirez A, Chan KY, de Vries R, Shook B, Jackson P, et al. Characterization of the trigeminovascular actions of several adenosine A2A receptor antagonists in an in vivo rat model of migraine. J Headache Pain. 2018;19:41.
Haanes KA, Edvinsson L. Expression and characterization of purinergic receptors in rat middle meningeal artery-potential role in migraine. PLoS One. 2014;9:e108782.
Fuller RW, Conradson TB, Dixon CM, Crossman DC, Barnes PJ. Sensory neuropeptide effects in human skin. Br J Pharmacol. 1987;92:781–8.
Pedersen-Bjergaard U, Nielsen LB, Jensen K, Edvinsson L, Jansen I, Olesen J. Calcitonin gene-related peptide, neurokinin A and substance P: effects on nociception and neurogenic inflammation in human skin and temporal muscle. Peptides. 1991;12:333–7.
Pedersen-Bjergaard U, Nielsen LB, Jensen K, Edvinsson L, Jansen I, Olesen J. Algesia and local responses induced by neurokinin A and substance P in human skin and temporal muscle. Peptides. 1989;10:1147–52.
Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30:1179–86.
Emery EC, Young GT, McNaughton PA. HCN2 ion channels: an emerging role as the pacemakers of pain. Trends Pharmacol Sci. 2012;33:456–63.
Young GT, Emery EC, Mooney ER, Tsantoulas C, McNaughton PA. Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels. Pain. 2014;155:1708–19.
Momin A, Cadiou H, Mason A, McNaughton PA. Role of the hyperpolarization-activated current Ih in somatosensory neurons. J Physiol. 2008;586:5911–29.
Tu H, Deng L, Sun Q, Yao L, Han JS, Wan Y. Hyperpolarization-activated, cyclic nucleotide-gated cation channels: roles in the differential electrophysiological properties of rat primary afferent neurons. J Neurosci Res. 2004;76:713–22.
Manteniotis S, Lehmann R, Flegel C, Vogel F, Hofreuter A, Schreiner BS, et al. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in trigeminal ganglia. PLoS One. 2013;8:e79523.
Cho HJ, Staikopoulos V, Furness JB, Jennings EA. Inflammation-induced increase in hyperpolarization-activated, cyclic nucleotide-gated channel protein in trigeminal ganglion neurons and the effect of buprenorphine. Neuroscience. 2009;162:453–61.
Oshinsky ML, Luo J. Neurochemistry of trigeminal activation in an animal model of migraine. Headache. 2006;46(Suppl 1):S39–44.
Vause CV, Durham PL. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J Neurochem. 2009;110:811–21.
Poolos NP, Bullis JB, Roth MK. Modulation of h-channels in hippocampal pyramidal neurons by p38 mitogen-activated protein kinase. J Neurosci. 2006;26:7995–8003.
Al-Karagholi MA, Hansen JM, Severinsen J, Jansen-Olesen I, Ashina M. The KATP channel in migraine pathophysiology: a novel therapeutic target for migraine. J Headache Pain. 2017;18:90.
Hogg RC, Adams DJ. An ATP-sensitive K(+) conductance in dissociated neurones from adult rat intracardiac ganglia. J Physiol. 2001;534:713–20.
Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–6.
Takasu K, Ono H, Tanabe M. Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain. Pain. 2010;151:87–96.
Manganiello VC, Degerman E. Cyclic nucleotide phosphodiesterases (PDEs): diverse regulators of cyclic nucleotide signals and inviting molecular targets for novel therapeutic agents. Thromb Haemost. 1999;82:407–11.
Humphrey PP. The discovery of a new drug class for the acute treatment of migraine. Headache. 2007;47(Suppl 1):S10–9.
Razzaque Z, Heald MA, Pickard JD, Maskell L, Beer MS, Hill RG, et al. Vasoconstriction in human isolated middle meningeal arteries: determining the contribution of 5-. Br J Clin Pharmacol. 1999;47:75–82.
Goadsby PJ, Edvinsson L. Joint 1994 Wolff Award Presentation. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C90. Headache. 1994;34:394–9.
Adham N, Romanienko P, Hartig P, Weinshank RL, Branchek T. The rat 5-hydroxytryptamine1B receptor is the species homologue of the human 5-hydroxytryptamine1D beta receptor. Mol Pharmacol. 1992;41:1–7.
Storer RJ, Goadsby PJ. Microiontophoretic application of serotonin (5HT)1B/1D agonists inhibits trigeminal cell firing in the cat. Brain. 1997;120(Pt 12):2171–7.
MaassenVanDenBrink A, van den Broek RW, de Vries R, Bogers AJ, Avezaat CJ, Saxena PR. Craniovascular selectivity of eletriptan and sumatriptan in human isolated blood vessels. Neurology. 2000;55:1524–30.
Longmore J, Hargreaves RJ, Boulanger CM, Brown MJ, Desta B, Ferro A, et al. Comparison of the vasoconstrictor properties of the 5-HT1D-receptor agonists rizatriptan (MK-462) and sumatriptan in human isolated coronary artery: outcome of two independent studies using different experimental protocols. Funct Neurol. 1997;12:3–9.
Rubio-Beltran E, Labastida-Ramirez A, Villalon CM, MaassenVanDenBrink A. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88–97.
Classey JD, Bartsch T, Goadsby PJ. Distribution of 5-HT(1B), 5-HT(1D) and 5-HT(1F) receptor expression in rat trigeminal and dorsal root ganglia neurons: relevance to the selective anti-migraine effect of triptans. Brain Res. 2010;1361:76–85.
Frederiksen SD, Warfvinge K, Ohlsson L, Edvinsson L. Expression of pituitary adenylate cyclase-activating peptide, calcitonin gene-related peptide and headache targets in the trigeminal ganglia of rats and humans. Neuroscience. 2018;393:319–32.
Shepheard S, Edvinsson L, Cumberbatch M, Williamson D, Mason G, Webb J, et al. Possible antimigraine mechanisms of action of the 5HT1F receptor agonist LY334370. Cephalalgia. 1999;19:851–8.
Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van BK, Mancuso V, et al. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci. 1997;61:2117–26.
Cohen ML, Schenck K. Contractile responses to sumatriptan and ergotamine in the rabbit saphenous vein: effect of selective 5-HT(1F) receptor agonists and PGF(2alpha). Br J Pharmacol. 2000;131:562–8.
Goldstein DJ, Roon KI, Offen WW, Ramadan NM, Phebus LA, Johnson KW, et al. Selective seratonin 1F (5-HT(1F)) receptor agonist LY334370 for acute migraine: a randomised controlled trial. Lancet. 2001;358:1230–4.
Ramadan NM, Skljarevski V, Phebus LA, Johnson KW. 5-HT1F receptor agonists in acute migraine treatment: a hypothesis. Cephalalgia. 2003;23:776–85.
Nelson DL, Phebus LA, Johnson KW, Wainscott DB, Cohen ML, Calligaro DO, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia. 2010;30:1159–69.
Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91:e2222–32.
Adham N, Kao HT, Schecter LE, Bard J, Olsen M, Urquhart D, et al. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci USA. 1993;90:408–12.
Labastida-Ramirez A, Rubio-Beltran E, Haanes KA, Danser J, Kovalchin J, Johnson KW, et al. Lasmiditan inhibits dural CGRP release from the rat trigeminovascular system. Cephalalgia. 2018;38(Suppl. 1):45–6.
Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338–50.
Schuster NM, Rapoport AM. Calcitonin gene-related peptide-targeted therapies for migraine and cluster headache: a review. Clin Neuropharmacol. 2017;40:169–74.
Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–10.
Ho TW, Connor KM, Zhang Y, Pearlman E, Koppenhaver J, Fan X, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83:958–66.
Voss T, Lipton RB, Dodick DW, Dupre N, Ge JY, Bachman R, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887–98.
Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia. 2014;34:114–25.
Tfelt-Hansen P, Loder E. The Emperor’s new gepants: are the effects of the new oral CGRP antagonists clinically meaningful? Headache. 2019;59:113–7.
Allergan. Efficacy, safety, and tolerability of multiple dosing regimens of oral atogepant (AGN-241689) in episodic migraine prevention [ClinicalTrials.gov identifier NCT02848326]. National Institutes of Health, ClinicalTrials.gov. 2019. https://clinicaltrials.gov. Accessed 2 Apr 2019.
Boado RJ, Zhou QH, Lu JZ, Hui EK, Pardridge WM. Pharmacokinetics and brain uptake of a genetically engineered bifunctional fusion antibody targeting the mouse transferrin receptor. Mol Pharm. 2010;7:237–44.
MaassenVanDenBrink A, Meijer J, Villalon CM, Ferrari MD. Wiping out CGRP: potential cardiovascular risks. Trends Pharmacol Sci. 2016;37:779–88.
Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltran E, et al. Blocking CGRP in migraine patients—a review of pros and cons. J Headache Pain. 2017;18:96.
Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113–22.
Lambru G, Andreou AP, Guglielmetti M, Martelletti P. Emerging drugs for migraine treatment: an update. Expert Opin Emerg Drugs. 2018. https://doi.org/10.1080/14728214.2018.1552939 (Epub 2018 Nov 28).
Zagami AS, Edvinsson L, Goadsby PJ. Pituitary adenylate cyclase activating polypeptide and migraine. Ann Clin Transl Neurol. 2014;1:1036–40.
Tighe AP, Schiavo G. Botulinum neurotoxins: mechanism of action. Toxicon. 2013;67:87–93.
Durham PL, Cady R. Insights into the mechanism of onabotulinumtoxinA in chronic migraine. Headache. 2011;51:1573–7.
Meng J, Wang J, Lawrence G, Dolly JO. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci. 2007;120:2864–74.
Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803.
Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–14.
Zhang X, Strassman AM, Novack V, Brin MF, Burstein R. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors’ responses to stimulation of TRPV1 and TRPA1 channels: are we getting closer to solving this puzzle? Cephalalgia. 2016;36:875–86.
Dolly JO, Wang J, Zurawski TH, Meng J. Novel therapeutics based on recombinant botulinum neurotoxins to normalize the release of transmitters and pain mediators. FEBS J. 2011;278:4454–66.
Mangione AS, Obara I, Maiaru M, Geranton SM, Tassorelli C, Ferrari E, et al. Nonparalytic botulinum molecules for the control of pain. Pain. 2016;157:1045–55.
Paraskevopoulou M, Perez JT, Miedzik A, Chamberlain J, Lambru G, Davletov B, et al. Non-paralytic botulinum molecules for the control of migraine. Cephalalgia. 2016;36 (Suppl 1):135.
Mustafa G, Anderson EM, Bokrand-Donatelli Y, Neubert JK, Caudle RM. Anti-nociceptive effect of a conjugate of substance P and light chain of botulinum neurotoxin type A. Pain. 2013;154:2547–53.
Maiaru M, Leese C, Certo M, Echeverria-Altuna I, Mangione AS, Arsenault J, et al. Selective neuronal silencing using synthetic botulinum molecules alleviates chronic pain in mice. Sci Transl Med. 2018;10:eaar7384.
Peroutka SJ. Neurogenic inflammation and migraine: implications for the therapeutics. Mol Interv. 2005;5:304–11.
Edvinsson L, Tajti J, Szalardy L, Vecsei L. PACAP and its role in primary headaches. J Headache Pain. 2018;19:21.
Tuka B, Helyes Z, Markovics A, Bagoly T, Szolcsanyi J, Szabo N, et al. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia. 2013;33:1085–95.
Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, et al. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166:4–17.
Chan KY, Baun M, de Vries R, van den Bogaerdt AJ, Dirven CM, Danser AH, et al. Pharmacological characterization of VIP and PACAP receptors in the human meningeal and coronary artery. Cephalalgia. 2011;31:181–9.
Amgen. Study to evaluate the efficacy and safety of AMG 301 in migraine prevention [ClinicalTrials.gov identifier NCT03238781]. National Institutes of Health, ClinicalTrials.gov. 2019. https://clinicaltrials.gov. Accessed 2 Apr 2019.
Moldovan LC, Dutzar B, Ojala EW, Hendrix L, Karasek C, Scalley-Kim M, et al. Pharmacologic characterization of ALD1910, a potent humanized monoclonal antibody against the pituitary adenylate cyclase activating peptide. J Pharmacol Exp Ther. 2019;369:26–36.
May V, Buttolph TR, Girard BM, Clason TA, Parsons RL. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am J Physiol Cell Physiol. 2014;306:C1068–79.
Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med. 2003;3:113–22.
Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal. 2012;1:789–803.
Queiroz G, Talaia C, Goncalves J. ATP modulates noradrenaline release by activation of inhibitory P2Y receptors and facilitatory P2X receptors in the rat vas deferens. J Pharmacol Exp Ther. 2003;307:809–15.
Guarracino JF, Cinalli AR, Fernandez V, Roquel LI, Losavio AS. P2Y13 receptors mediate presynaptic inhibition of acetylcholine release induced by adenine nucleotides at the mouse neuromuscular junction. Neuroscience. 2016;326:31–44.
Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost. 2001;86:222–32.
Haanes KA, Labastida-Ramirez A, Dirven CM, Danser AHJ, MaassenVanDenBrink A. Purinergic receptors as potential anti-migraine targets. Cephalalgia. 2016;36(Suppl. 1):140.
Wang L, Burmeister BT, Johnson KR, Baillie GS, Karginov AV, Skidgel RA, et al. UCR1C is a novel activator of phosphodiesterase 4 (PDE4) long isoforms and attenuates cardiomyocyte hypertrophy. Cell Signal. 2015;27:908–22.
McNaughton P (2018) HCN2 ion channels- a new target in migraine? MTIS London, Novel Transmitter Systems, 8th Septhember 2018.
Alshammari TM. Ivabradine: do the benefits outweigh the risks? J Cardiovasc Pharmacol Ther. 2017;22:210–8.
Baruscotti M, Bucchi A, Viscomi C, Mandelli G, Consalez G, Gnecchi-Rusconi T, et al. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc Natl Acad Sci USA. 2011;108:1705–10.
Lee CH, MacKinnon R. Structures of the human HCN1 hyperpolarization-activated channel. Cell. 2017;168:111–20.
Novella RM, Sartiani L, Masi A, Mannaioni G, Manetti D, Mugelli A, et al. HCN channels modulators: the need for selectivity. Curr Top Med Chem. 2016;16:1764–91.
Xiao Y, Richter JA, Hurley JH. Release of glutamate and CGRP from trigeminal ganglion neurons: Role of calcium channels and 5-HT1 receptor signaling. Mol Pain. 2008;4:12.
Schneider SP, Perl ER. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. J Neurosci. 1988;8:2062–73.
Sang CN, Ramadan NM, Wallihan RG, Chappell AS, Freitag FG, Smith TR, et al. LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia. 2004;24:596–602.
Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014;74:1165–76.
Waung MW, Akerman S, Wakefield M, Keywood C, Goadsby PJ. Metabotropic glutamate receptor 5: a target for migraine therapy. Ann Clin Transl Neurol. 2016;3:560–71.
Curto M, Lionetto L, Fazio F, Mitsikostas DD, Martelletti P. Fathoming the kynurenine pathway in migraine: why understanding the enzymatic cascades is still critically important. Intern Emerg Med. 2015;10:413–21.
Stone DR, Downs JB, Paul WL, Perkins HM. Adult body temperature and heated humidification of anesthetic gases during general anesthesia. Anesth Analg. 1981;60:736–41.
Fejes-Szabo A, Bohar Z, Vamos E, Nagy-Grocz G, Tar L, Veres G, et al. Pre-treatment with new kynurenic acid amide dose-dependently prevents the nitroglycerine-induced neuronal activation and sensitization in cervical part of trigemino-cervical complex. J Neural Transm (Vienna). 2014;121:725–38.
Lukacs M, Haanes KA, Majlath Z, Tajti J, Vecsei L, Warfvinge K, et al. Dural administration of inflammatory soup or Complete Freund’s Adjuvant induces activation and inflammatory response in the rat trigeminal ganglion. J Headache Pain. 2015;16:564.
Lukacs M, Warfvinge K, Tajti J, Fulop F, Toldi J, Vecsei L, et al. Topical dura mater application of CFA induces enhanced expression of c-fos and glutamate in rat trigeminal nucleus caudalis: attenuated by KYNA derivate (SZR72). J Headache Pain. 2017;18:39.
Cameron C, Kelly S, Hsieh SC, Murphy M, Chen L, Kotb A, et al. Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache. 2015;55(Suppl 4):221–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lars Edvinsson has given lectures on CGRP for Amgen, Novartis, and Teva, and has received minor grant support, though none pertaining to the current manuscript. Kristian Agmund Haanes has no conflicts of interest to report.
Funding
No sources of funding were used to assist with the preparation of this review.
Rights and permissions
About this article
Cite this article
Haanes, K.A., Edvinsson, L. Pathophysiological Mechanisms in Migraine and the Identification of New Therapeutic Targets. CNS Drugs 33, 525–537 (2019). https://doi.org/10.1007/s40263-019-00630-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00630-6