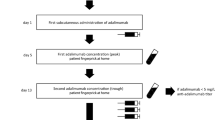
Abstract
Background and Objectives
The pharmacokinetics of infliximab are highly variable and influence clinical response in chronic inflammatory diseases. The goal of this study was to build a Bayesian model allowing predictions of upcoming infliximab concentrations and dosing regimen adjustment, using only one concentration measurement and information regarding the last infliximab infusion.
Methods
This retrospective study was based on data from 218 patients treated with infliximab in Tours University Hospital who were randomly assigned to learning (two-thirds) or validation (one-third) data subsets. One-compartment pharmacokinetic and time since last dose (TLD) models were built and compared using learning and validation subsets. From these models, Bayesian pharmacokinetic and TLD models using one concentration measurement (1C-PK and 1C-TLD) were designed. The predictive performances of the 1C-TLD model were tested on two external validation cohorts.
Results
Pharmacokinetic and TLD models described the data satisfactorily and provided accurate parameter estimations. Comparable predictions of infliximab concentrations were obtained from pharmacokinetic versus TLD models, as well as from Bayesian 1C-PK versus 1C-TLD models. The 1C-TLD model showed satisfactory prediction of future infliximab concentrations and provided satisfactory predictions of infliximab steady-state concentration for up to three upcoming visits after a blood sample.
Conclusions
Accurate individual concentration predictions can be obtained using a single infliximab concentration measurement and information regarding only the last infusion. The 1C-TLD model may help to optimize the dosing regimen of infliximab in routine therapeutic drug monitoring.





Similar content being viewed by others
References
Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348(7):601–8.
Krzysiek R, Breban M, Ravaud P, et al. Circulating concentration of infliximab and response to treatment in ankylosing spondylitis: results from a randomized control study. Arthritis Rheumatol. 2009;61(5):569–76.
Passot C, Mulleman D, Bejan-Angoulvant T, et al. The underlying inflammatory chronic disease influences infliximab pharmacokinetics. MAbs. 2016;8(7):1407–16.
Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–74.
St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2002;46(6):1451–9.
Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheumatol. 1998;41(9):1552–63.
Mulleman D, Lin DCM, Ducourau E, et al. Trough infliximab concentrations predict efficacy and sustained control of disease activity in rheumatoid arthritis. Ther Drug Monit. 2010;32(2):232–6.
Mulleman D, Meric JC, Paintaud G, et al. Infliximab concentration monitoring improves the control of disease activity in rheumatoid arthritis. Arthritis Res Ther. 2009;11(6):R178 epub 2009 Nov 25.
Wolbink GJ, Voskuyl AE, Lems WF, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64(5):704–7.
Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4(10):1248–54 epub 2006 Aug 22.
van den Bemt BJ, den Broeder AA, Wolbink GJ, et al. The combined use of disease activity and infliximab serum trough concentrations for early prediction of (non-)response to infliximab in rheumatoid arthritis. Br J Clin Pharmacol. 2013;76(6):939–45.
Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63(11):1721–7.
Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147(6):1296–307.
Steenholdt C, Brynskov J, Thomsen OO, et al. Individualized therapy is a long-term cost-effective method compared to dose intensification in Crohn’s disease patients failing infliximab. Dig Dis Sci. 2015;60(9):2762–70.
Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320–9.
Medina F, Plasencia C, Goupille P, et al. Current practice for therapeutic drug monitoring of biopharmaceuticals in rheumatoid arthritis. Ther Drug Monit. 2017;39(4):364–9.
Brandse JF, Mould D, Smeekes O, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(4):650–60.
Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20(12):2247–59.
Mould DR, Dubinsky MC. Dashboard systems: pharmacokinetic/pharmacodynamic mediated dose optimization for monoclonal antibodies. J Clin Pharmacol. 2015;2015(55 Suppl. 3):S51–9.
Aubourg A, Picon L, Lecomte T, et al. A robust estimation of infliximab pharmacokinetic parameters in Crohn’s disease. Eur J Clin Pharmacol. 2015;71(12):1541–2.
Bejan-Angoulvant T, Ternant D, Daoued F, et al. Brief report: relationship between serum infliximab concentrations and risk of infections in patients treated for spondyloarthritis. Arthritis Rheumatol. 2017;69(1):108–13.
Ternant D, Aubourg A, Magdelaine-Beuzelin C, et al. Infliximab pharmacokinetics in inflammatory bowel disease patients. Ther Drug Monit. 2008;30(4):523–9. https://doi.org/10.1097/FTD.0b013e318180e300.
Ternant D, Mulleman D, Degenne D, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther Drug Monit. 2006;28(2):169–74.
Ternant D, Ducourau E, Perdriger A, et al. Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis. Br J Clin Pharmacol. 2014;78(1):118–28.
Ternant D, Mulleman D, Lauféron F, et al. Influence of methotrexate on infliximab pharmacokinetics and pharmacodynamics in ankylosing spondylitis. Br J Clin Pharmacol. 2011;73(1):55–65.
Passot C, Pouw MF, Mulleman D, et al. Therapeutic drug monitoring of biopharmaceuticals may benefit from pharmacokinetic and pharmacokinetic-pharmacodynamic modeling. Ther Drug Monit. 2017;39(4):322–6.
Fasanmade AA, Adedokun OJ, Blank M, et al. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33(7):946–64.
Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65(12):1211–28.
Xu Z, Seitz K, Fasanmade A, et al. Population pharmacokinetics of infliximab in patients with ankylosing spondylitis. J Clin Pharmacol. 2008;48(6):681–95.
Benkali K, Premaud A, Picard N, et al. Tacrolimus population pharmacokinetic-pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin Pharmacokinet. 2009;48(12):805–16. https://doi.org/10.2165/11318080-000000000-00000.
Kassir N, Labbe L, Delaloye JR, et al. Population pharmacokinetics and Bayesian estimation of tacrolimus exposure in paediatric liver transplant recipients. Br J Clin Pharmacol. 2014;77(6):1051–63.
Thomson AH, Whiting B. Bayesian parameter estimation and population pharmacokinetics. Clin Pharmacokinet. 1992;22(6):447–67.
Zhao W, Elie V, Baudouin V, et al. Population pharmacokinetics and Bayesian estimator of mycophenolic acid in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol. 2010;69(4):358–66.
Dreesen E, Gils A, Vermeire S. Pharmacokinetic modeling and simulation of biologicals in inflammatory bowel disease: the dawning of a new era for personalized treatment. Curr Drug Targets. (epub 2016 Mar 7).
Sheiner LB, Beal SL. Bayesian individualization of pharmacokinetics: simple implementation and comparison with non-Bayesian methods. J Pharm Sci. 1982;71(12):1344–8.
Yamamoto T, Terakawa H, Hisaka A, et al. Bayesian estimation of pharmacokinetic parameters of vancomycin in patients with decreasing renal function. J Pharm Sci. 2012;101(8):2968–75.
FDA. Guidance for industry: population pharmacokinetics. Rockville (MD): CDER; 1999. https://www.fda.gov/downloads/drugs/guidances/UCM072137.pdf. Accessed 1 Jun 2017.
Mulleman D, Ducourau E, Paintaud G, et al. Should anti-TNF-alpha drug levels and/or anti-drug antibodies be assayed in patients treated for rheumatoid arthritis? Jt Bone Spine. 2012;79(2):109–12.
Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21(1):182–97.
Ternant D, Berkane Z, Picon L, et al. Assessment of the influence of inflammation and FCGR3A genotype on infliximab pharmacokinetics and time to relapse in patients with Crohn’s disease. Clin Pharmacokinet. 2015;54(5):551–62.
Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014;63(8):1258–64.
Ng CM, Loyet KM, Iyer S, et al. Modeling approach to investigate the effect of neonatal Fc receptor binding affinity and anti-therapeutic antibody on the pharmacokinetic of humanized monoclonal anti-tumor necrosis factor-alpha IgG antibody in cynomolgus monkey. Eur J Pharm Sci. 2014;51:51–8.
Acknowledgements
The authors thank Drs. Saloua Mammou, Stéphanie Willot, Mahtab Samimi, and Annabel Maruani for patient follow-up; Anne-Claire Duveau and Caroline Guerineau-Brochon for technical assistance with infliximab assays; and the medical staff and nurses from the Rheumatology, Gastroenterology, Dermatology, and Paediatrics departments.
Author information
Authors and Affiliations
Contributions
DT analyzed and interpreted the data, and wrote the manuscript. CP participated in the data conception, analysis, and interpretation and reviewed the manuscript. TL participated in data conception and interpretation, and reviewed the manuscript. CD participated in data conception and reviewed the manuscript. NA participated in data analysis and reviewed the manuscript. AA, LP, and PG participated in data acquisition and reviewed the manuscript. DM participated in data acquisition and interpretation, and reviewed the manuscript. GP supervised the study, participated in the writing of the manuscript, and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Informed Consent
Ethical approval and informed consent were not sought in this retrospective analysis of routine patients, which is in accordance with institutional guidelines.
Funding
Measurements of infliximab serum concentrations were carried out within the CePiBAc (Centre Pilote de suivi Biologique des traitements par Anticorps) platform, which was co-financed by the European Union and the European Regional Development Fund. This work was partly supported by the French Higher Education and Research Ministry under the program ‘investissements d’avenir’ (Grant agreement: LabEx MAbImprove ANR-10-LABX-53-01). CNRS UMR 7292 participates in the Monitoring of monoclonal Antibodies Group in Europe (MAGE) Consortium for inflammatory diseases. The MAGE Consortium is supported by LE STUDIUM Loire Valley Institute for Advanced Studies (http://www.lestudium-ias.com).
Conflict of interest
David Ternant has given lectures on behalf of his institution for Amgen and Sanofi. Alexandre Aubourg has acted as a consultant for MSD, Abbvie, Takeda, and Janssen. He has also received support for travel by MSD, Ferring, and Abbvie. Philippe Goupille has been a consultant for Abbvie, Biogaran, BMS, Celgene, Janssen, MSD, Novartis, Pfizer, and UCB; he has participated on behalf of his institution in clinical trials sponsored by Abbvie, Biogaran, BMS, Boehringer, Janssen, Lilly, MSD, Novartis, Pfizer, and UCB. Denis Mulleman has participated on behalf of his institution in clinical trials sponsored by Abbott, Roche, BMS, Pfizer, UCB, and MSD; his hospital department received a grant for research from Abbott in 2004 and from Nordic Pharma in 2012; he has acted as a consultant and given lectures on behalf of his institution for MSD and Pfizer; and he has been invited to attend international congresses by MSD, Roche, BMS, Abbott, and Janssen-Cilag. Gilles Paintaud has received grants for his research team from Roche Pharma, Chugai, Pfizer, Novartis, and Sanofi-Genzyme. Christophe Passot, Thierry Lecomte, Laurence Picon, and Céline Desvignes have nothing to declare.
Rights and permissions
About this article
Cite this article
Ternant, D., Passot, C., Aubourg, A. et al. Model-Based Therapeutic Drug Monitoring of Infliximab Using a Single Serum Trough Concentration. Clin Pharmacokinet 57, 1173–1184 (2018). https://doi.org/10.1007/s40262-017-0621-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0621-6